Question
Question 1 Evaluate the global pharmaceutical industry position with Porter five forces framework. Question 2 Discuss the PESTLE environmental forces that affect the global pharmaceutical

Question 1
Evaluate the global pharmaceutical industry position with Porter five forces framework.
Question 2
Discuss the PESTLE environmental forces that affect the global pharmaceutical industry. Cite on relevant issues from the case to support your answer.
Question 3
Describe how has the strategic customer evolved over time for the global pharmaceutical companies and explain its impact on the industrys critical success factors.
Question 4
According to Kindler, he faces the legacy of blockbuster business model and his company was focused on conventional medicines at a time of increased regulatory scrutiny and declining of R&D productivity. Advise him FOUR (4) possible strategic changes that need to be implemented.
Question 5
Conduct a SWOT analysis based on the case as mentioned.
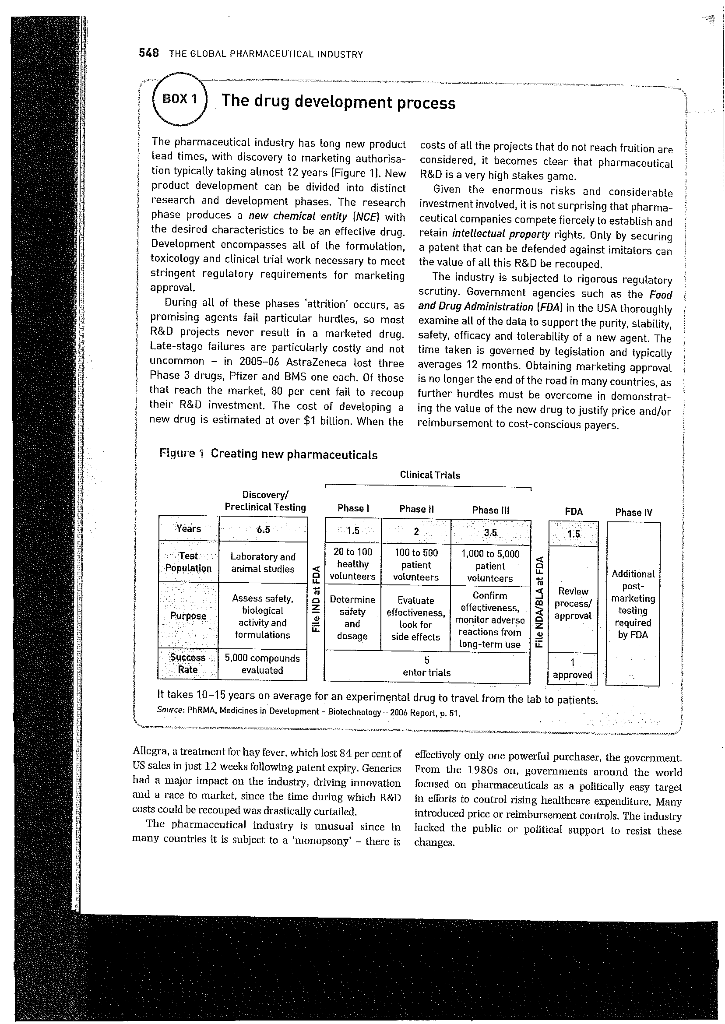
CASE STUDY The global pharmaceutical industry: swallowing a bitter pill Sarah Holland The case describes the evolution of the pharmaceutical industry and its unusual strategic environment. Attention is drawn to environmental pressures from regulators and payers. Key forces driving the industry are discussed. including addressing unmet medical needs, the importance of innovation and tire to market, and globalisation. The case illustrates how an increasingly hostilo cnvironment combined with a decline in R&D productivity, led to waves of job losses, and sparked a fresh round of consolidation in the industry. On the global level, the historical supremacy of the US was being challenged with the highest market growth rates recorded emerging markets. The case is designed to facilitate teaching of analysis frameworks including PESTEL, Porter's five forces, the concept of the strategic customer' and industry critical success factors. It may also be used for stakeholder analysis and as a basis for discussion of social responsibility INSIGNING A CEO's dilemma development (R&D) process, intense competition for On 23 September 2008, Pliner CEO Jeff Kindler took to intellectual property. stringent government regulation and powerful purchaser pres. How has this masual the stage at the World Business Forum to be interviewed by Pox News anchor Liz Claymun, Pfizer Weslle world's picture Comes boul? number 1 pharmaceutical company with $13 billion The origins of the modern pharmaceutical industry can (4.5ba or 18.6bn) in annual recics from its block- be traced to the late nineleenit century, when dyestutt's buster cholesterol-lowering drug Lipitor. Contributing were found to have antiseptic propertics. Penicillin Was it major discovery, and R&D become firmly established within almost u third of company turnover, Lipicar facxxl patent expiry with dramatic loss of sales value in 2011. A key the tour. The market developed some anasual charac- drug intended replace it had lailed to late-stage clinical teristics. Decision making was in the hands of medical testing and investors were losing confidence, Claymun practttioners whereas palierils (the final consumers) and wanted know how Kindler planned to keep Plzer afloat. payers (governments or insurance companies) lad lille Acknowledging that no one drug could replace lipitur, knowledge or influence. Conscqucntly, meclical practitioners Kindler described Pitzer's broud pipeline of new drugs and were inscnsitive price but susceptible to the cfforts of 'very strong balance sheet und significant amount of cash'. sales representatives Kindler ce lie legacy of the blockbuster business reoddel, Two Important developments accurred in the 1970s. and his company was focused on conventional medicines Firstly, ita thalidomide tragedy (an antiemetic for morn- at a time of increased regulatory scrutiny and declining ing sickness that caused birth defect) led lo much lighter regulatory conlrols clinical trials. Secondly, legislation K&D productivity. Something needed to change, but what? was enacted to set a fixed period on patent pruleclion - typically 20 ycars from initial filing. On patent expiry, Industry evolution rivels could launch generic medicines with exactly the As described in Box 1. the pharmaceutical industry is same active ingredients is the riginal brand. ut a lower characterised by a highly risky and lengthy research and price. The dramatic impact of generic entry is thustrated by Terme given in bold falte urc dclined in the Clussury At the end of the case. +51 - 60.73 or 0.66. This case prepared by Sarah Holland. It is intended as a basis for class discussion and not as on illustration of good or bad prac- tice. O KS. Holland 2010. Not to be reproduced or quoted without permission. 548 THE GLOBAL PHARMACEUTICAL INDUSTRY BOX 1 The drug development process The pharmaceutical industry has long new product costs of all the projects that do not reach fruition are lead times, with discovery to marketing authorisa- considered, it becomes clear that pharmacoutical tion typically taking alinost 12 years (Figure 1). New R&D is a very high stakes game. product development can be divided into distinct Given the enormous risks and considerable research and development phases. The research investment involved, it is not surprising that pharma- phase produces a new chemical entity (NCE) with ceutical companies compete fiercely lo establish and the desired characteristics to be an effective drug. retain intellectual proporty rights. Only by securing Development oncompasses all of the formulation, a patent that can be defended against imitators can toxicology and clinical trial work necessary to meet the value of all this R&D be recouped. stringent regulalory requirements for marketing The industry is subjected lo rigorous regulatory scrutiny. Government agencies such as the Food During all of these phases 'attrition' occurs, as and Drug Administration (FDA) in the USA Loroughly promising agents fail particular hurdles, so most examine all of the data to support the purity, stability, R&D projects never result in a marketed drug. safety, officacy and tolerability of a new agent. The Late-stago failures are particularly costly and not time taken is governed by legislation and typically uncommon - in 2005-06 AstraZeneca lost three averages 12 months. Obtaining marketing approval Phase 3 drugs, Pfizer and BMS one cach. Of those is no longer the end of the road in many countries, as that reach the inarket, 80 por cent fail to recoup further hurdles must be overcome in demonstrat- their R&D investment. The cost of developing a ing the value of the new drug to justify price and/or new drug is estimated at over $1 billion. When the reimbursement to cost-conscious payers. approval Figure : Creating new pharmaceuticals Clinical Trials Discovery/ Preclinical Testing Phase 1 Phase Phasa ili FDA Phase IV Years 6.5 1.5 . 1.5 ":"Test Population Laboratory and artimal studies 20 to 100 healthy volunteers Review process/ 2 3.5. 100 to 500 1,000 to 5,000 patient patient volunteers volunteers Confirm Evaluate elfactiveness, effectiveness look for monitor adverso reactions froin side effects long-term use 5 entor trials Assess safely. biological activity and formulations e Dotermine safety and dosage Purpose Additional post- marketing testing required by FDA approval Success Rate 5,000 compounds evaluated approved It takes 10-15 years on average for an experimental drug to travel from the lab to patients. Snice: PhRMA, Medicinos in Development - Biotechnology. 2006 Report, u. 51, .... Allegra, a treatment for hay fever, which lost 81 per cent of electivoly only cwe powerful purchaser, the govcrument US sales in just 12 weeks following pulent expiry. Generics from the 1980s ou, governments around the world had a major impact on the industry, driving innovation focused on pharmaceuticals as a politically easy target ud a mice to turket, since the time during which RX12 in elleris to coutrol rising healthcare expenditure. Many usts could be recouped was cirastically curlailed. introduced price or reimbursement Controls. The industry The pharmaceutical Industry is unusual since In fucked the public or political support to resist these many countries it is subject to a mumlupsony' - there is changes
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


