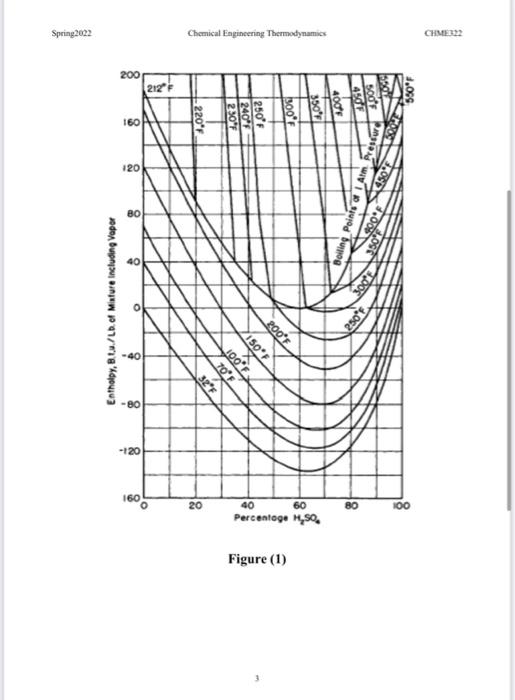
Question 1 Nitrobenzene is made by the direct nitration of benzene by nitric acid in the presence of strong sulphuric acid solution: CH + HNO, CH.NO, +H,0 The actual nitrating agent is the nitronium ion, NO2", and the sulphuric acid is needed to generate this from the nitric acid feed. Reconcentrated sulphuric acid, of strength 74% by weight and XC is mixed with pure nitric acid at 25C to give a nitric acid strength in the mixture of 7.5% by weight. The heat of mixing causes a temperature rise to 95 C. The mixed acid is then passed through a heat exchanger to bring its final temperature to 65 C and fed to the reactor. Liquid benzene at 25C is also fed to the reactor at a rate to give a 10% stoichiometric excess based on the nitric acid flow. The reactor operates adiabatically and gives 100% conversion of the nitric acid. All products leave the reactor as liquids at Y'C. The sulphuric acid is re-concentrated to 74% and recycled, while the nitrobenzene is sent for purification. Using the attached thermodynamic data, you are asked to calculate: (i) the temperature, XC of the reconcentrated sulphuric acid (ii) the amount of water removed to bring the concentration of the sulphuric acid solution back to 74% (iii) the temperature, Y'C, at the exit of the reactor (iv) the heat load on the heat exchanger per kg of nitrobenzene produced. Discussions of the overall results and what you have learnt. You may assume that the organics benzene and nitrobenzene form an ideal liquid mixture, and there is complete immiscibility between organics and inorganics. 49.00 -285.83 Spring2022 Chemical Engineering Thermodynamics CHME322 Thermodynamic data a Heats of Formation Compound AH, MJ kmol CH/1) CH:NO: (1) 1252 HNO (1) - 173.35 H:0 (1) Specific heat capacities of the organics For benzene: -0.2837 -2.7579 x 1OT (C is in kcal kg'K' and T in K) For nitrobenzene: T 20 30 40 50 60 70 SO 90 100 110 120 130 140 C 1.468 1.479 1.495, 1.514 1.535 1.559 1.584 1.610 1.637 1.664 1.691 0.718 1.745 (in kJ kg 'K', Tin C) c Thermodynamic data for the inorganics A. The binary system H:0-H:SO: Figure (1) B. The ternary system 1:0 - HNO: - H:SO4 Enthalpy (Kcal kg) at 25C HNO, (wt%) 0 5 10 15 H:SO. (wt%) 60 -773 -76.7 -735-71.3 -76.4 -745 -725 -20.3 70 75.6 -72.8 60.2 75 74.7 -65.8 -63.0 58.6 65 -670 The specific heat capacity of the mixture in the form: C, -2+bat (where tis in "C) a (kcal 1) bx10(Kcal kg HNO HNO, H,SO (wt%) . 3 10 15 H.SO (t) .s 10 15 60 60 33 -0.4 -0% WE 65 35 65 70 75 ARA 0.511 0.482 0.463 0.451 0.481 0.476 0.460 0.449 0.4540.448 0.436 0.428 0.435 0.423 0.419 -15 -1.8 70 3.6 27 0.410 75 30 -07 Spring 2002 Chemical Engineering Thermodynamics CHMER2 200 212 550F 160 -220F Pressure 120 IA 80 Boiling Points 40 Enthalpy, B.u./Lb. of Minture Including Vapor 250" 200F 40 %150F 32F 1.02 1,001 80 -120 160 20 80 100 40 60 Percentage M,so ! Figure (1)