Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 11 1 Point If you have two solutions X and Y. Solution X contains an unknown acid, HX, and solution Y contains an unknown
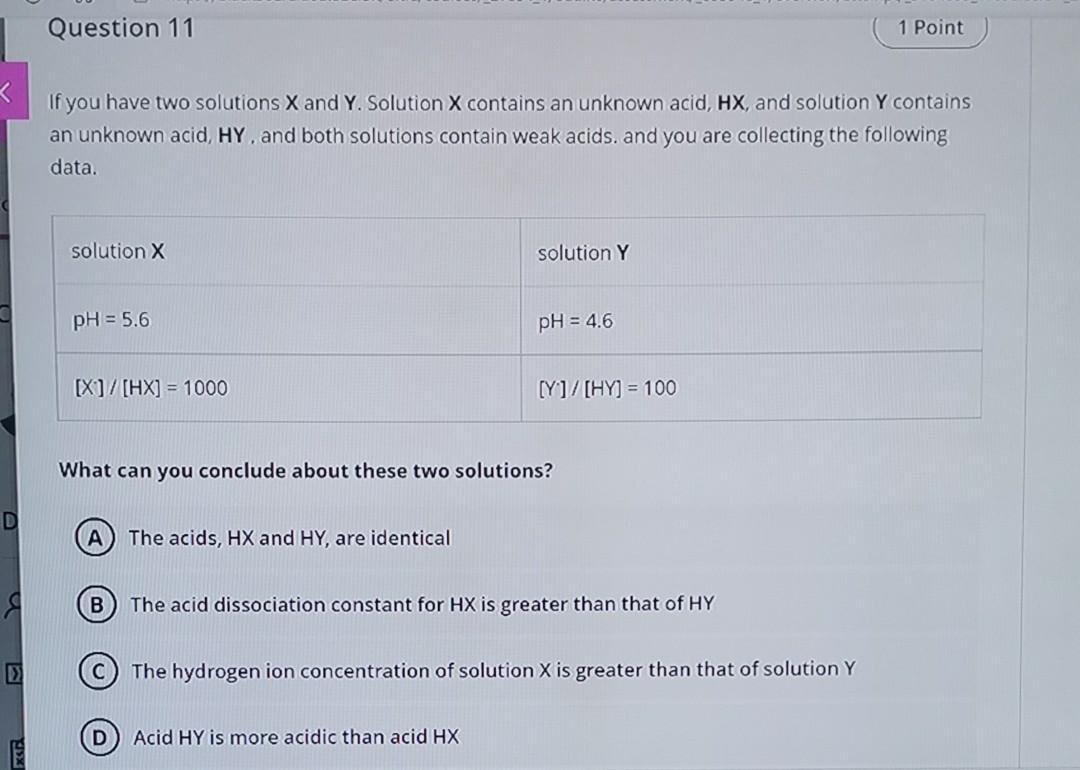
Question 11 1 Point If you have two solutions X and Y. Solution X contains an unknown acid, HX, and solution Y contains an unknown acid, HY. and both solutions contain weak acids, and you are collecting the following data. solution X solution Y pH = 5.6 pH = 4.6 [X]/[HX] = 1000 [Y]/[HY] = 100 What can you conclude about these two solutions? A) The acids, HX and HY, are identical B The acid dissociation constant for HX is greater than that of HY The hydrogen ion concentration of solution X is greater than that of solution Y D Acid HY is more acidic than acid HX
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


