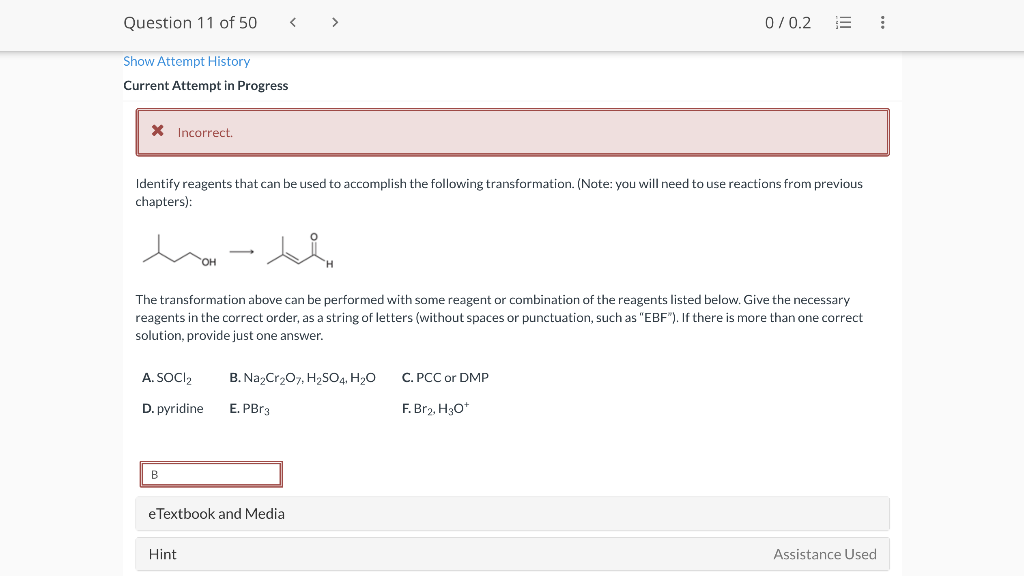
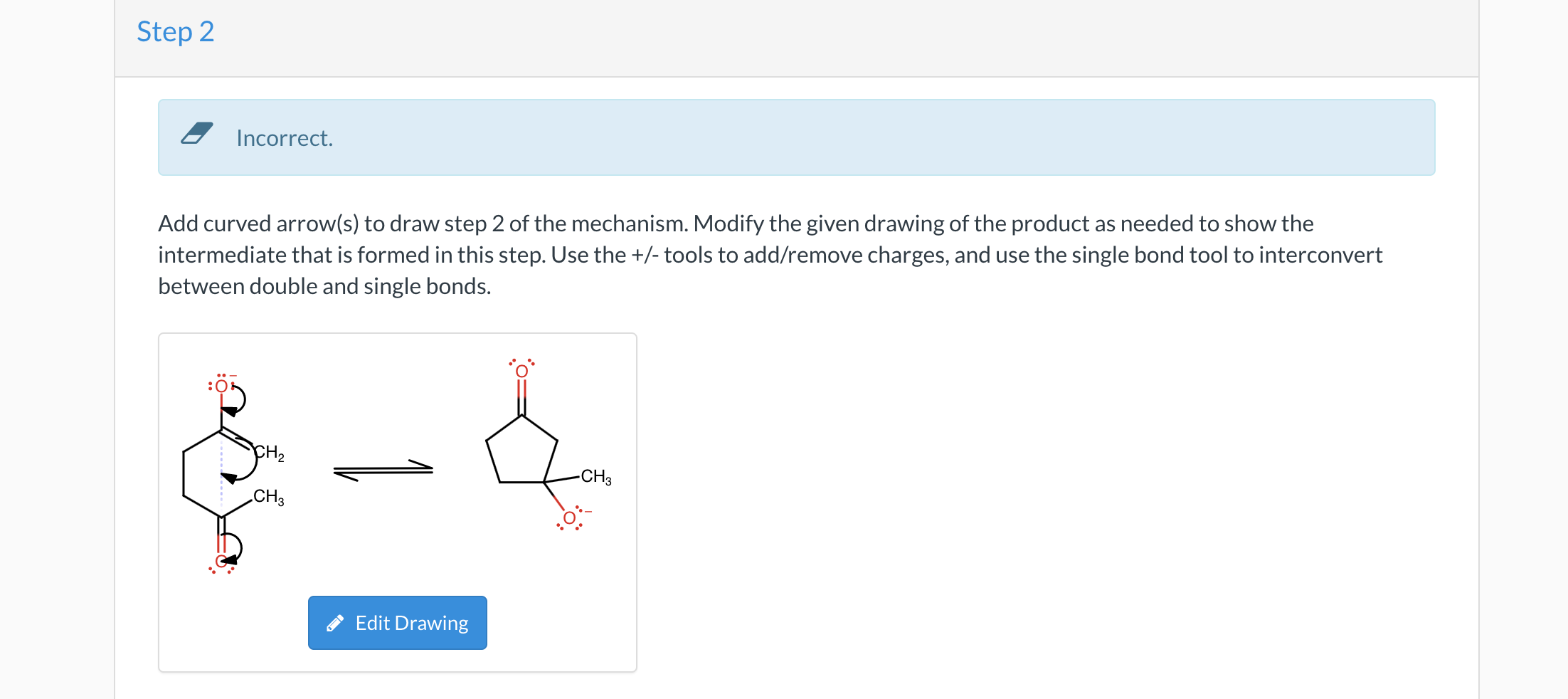
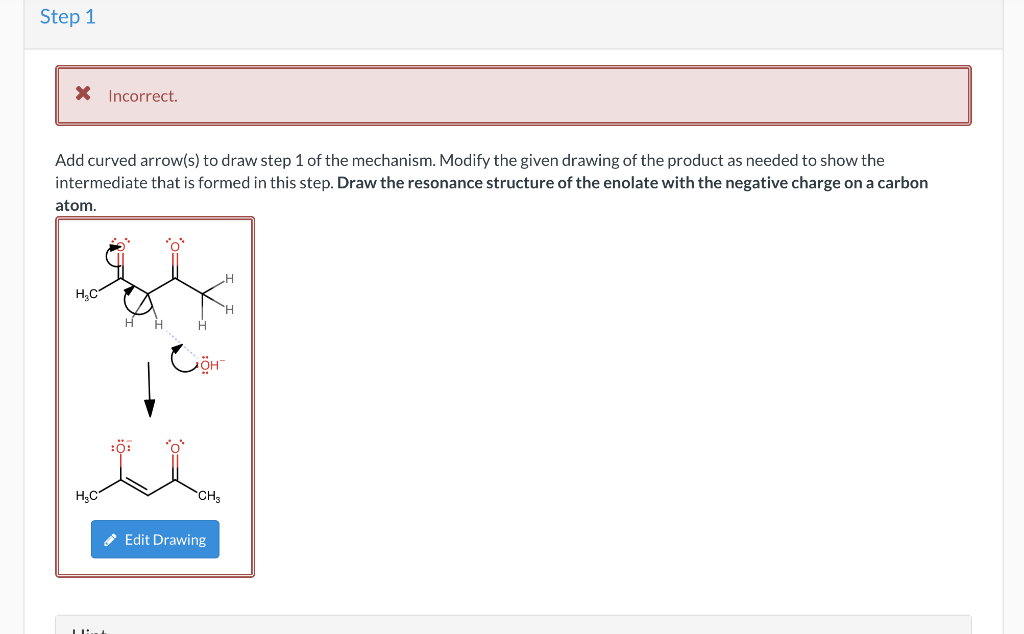

Question 11 of 50 0/0.2 III : Show Attempt History Current Attempt in Progress X Incorrect. Identify reagents that can be used to accomplish the following transformation. (Note: you will need to use reactions from previous chapters): hasi The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A. SOCI2 B. Na2Cr207, H2SO4, H20 C. PCC or DMP D. pyridine E. PBrg F. Br2, H307 B e Textbook and Media Hint Assistance Used Step 2 Incorrect. Add curved arrow(s) to draw step 2 of the mechanism. Modify the given drawing of the product as needed to show the intermediate that is formed in this step. Use the +/- tools to add/remove charges, and use the single bond tool to interconvert between double and single bonds. CH -CH3 CHZ Edit Drawing Step 1 X Incorrect Add curved arrow(s) to draw step 1 of the mechanism. Modify the given drawing of the product as needed to show the intermediate that is formed in this step. Draw the resonance structure of the enolate with the negative charge on a carbon atom. H.C . HC CH Edit Drawing (b) When the enamine(s) formed in part a is (are) treated with methyl iodide, followed by aqueous acid, 2-methylcyclohexanone with 83% ee is isolated. Select the reasons why the % ee achieved in this reaction sequence is considerably higher than that achieved in the reaction sequence presented in the previous problem (22.110). Since this is the only enamine undergoing alkylation due to the rotational symmetry of the secondary amine, the R- enantiomer is the major product formed. Since approach from the bottom face of the enamine is unimpeded, only (5)-2-methylcyclohexanone forms. Since approach from the bottom face of the enamine is unimpeded, only (R)-2-methylcyclohexanone forms. Since approach from the top face of the enamine is unimpeded, only (s)-2-methylcyclohexanone forms. The methyl group of the enamine close to the alpha carbon is below the plane preventing approach of the methyl iodide from below the enamine. The methyl group of the enamine close to the alpha carbon is above the plane preventing approach from the methy iodide from above the enamine. Since this is the only enamine undergoing alkylation due to the rotational symmetry of the secondary amine, the S- enantiomer is the major product formed. Since approach from the top face of the enamine is unimpeded, only (R)-2-methylcyclohexanone forms. Rotational symmetry of the enamine is not responsible for the %ee achieved. Rotational symmetry of the enamine is responsible for the %ee achieved










