Answered step by step
Verified Expert Solution
Question
1 Approved Answer
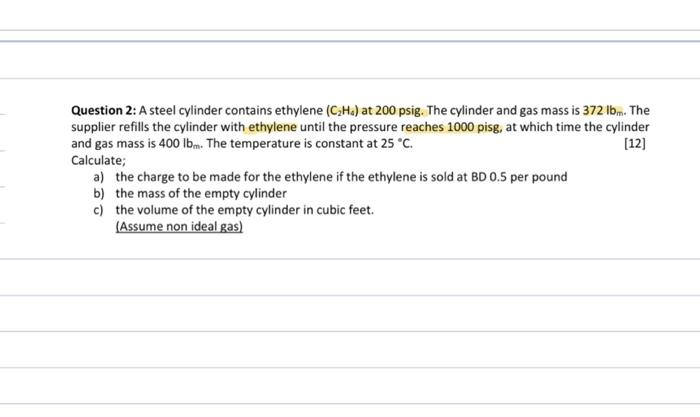
Question 2: A steel cylinder contains ethylene (CH4) at 200 psig. The cylinder and gas mass is 372 lbm. The supplier refills the cylinder with
Question 2: A steel cylinder contains ethylene (CH4) at 200 psig. The cylinder and gas mass is 372 lbm. The supplier refills the cylinder with ethylene until the pressure reaches 1000 pisg, at which time the cylinder and gas mass is 400 lbm. The temperature is constant at 25 C. [12] Calculate; a) the charge to be made for the ethylene if the ethylene is sold at BD 0.5 per pound b) the mass of the empty cylinder c) the volume of the empty cylinder in cubic feet. (Assume non ideal gas)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


