Answered step by step
Verified Expert Solution
Question
1 Approved Answer
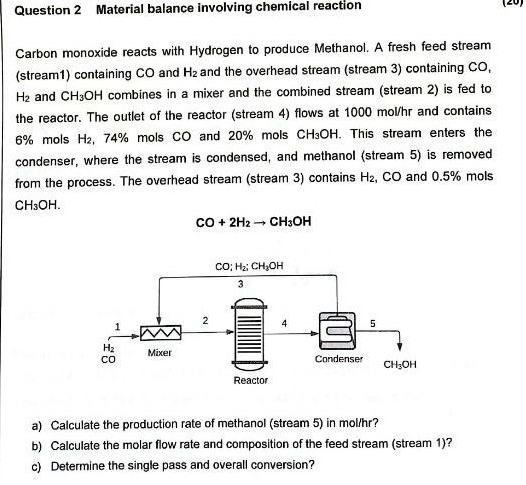
Question 2 Material balance involving chemical reaction Carbon monoxide reacts with Hydrogen to produce Methanol. A fresh feed stream ( stream 1 ) containing C
Question Material balance involving chemical reaction
Carbon monoxide reacts with Hydrogen to produce Methanol. A fresh feed stream stream containing and and the overhead stream stream containing and combines in a mixer and the combined stream stream is fed to the reactor. The outlet of the reactor stream flows at and contains mols mols and mols This stream enters the condenser, where the stream is condensed, and methanol stream is removed from the process. The overhead stream stream contains and mols
a Calculate the production rate of methanol stream in molhr
b Calculate the molar flow rate and composition of the feed stream stream
c Determine the single pass and overall conversion?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


