It is known that the pressure and temperature of a storage tank that stores the cyclohexane vapor are 16.292 bar and 719.68 K. The
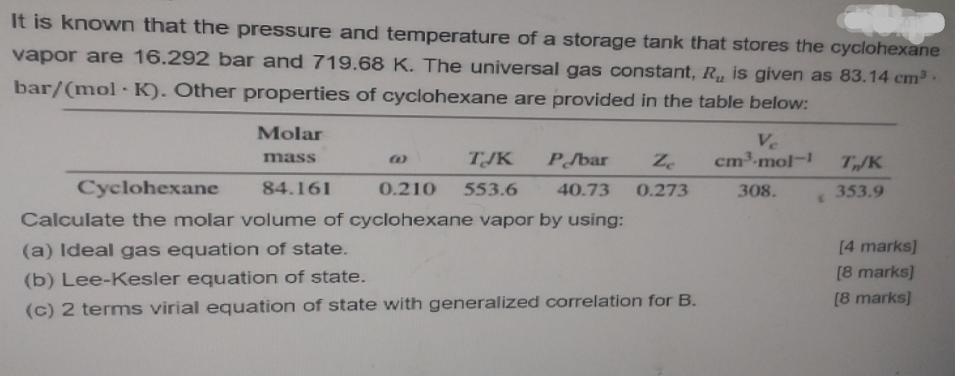
It is known that the pressure and temperature of a storage tank that stores the cyclohexane vapor are 16.292 bar and 719.68 K. The universal gas constant, R, is given as 83.14 cm bar/(mol- K). Other properties of cyclohexane are provided in the table below: Molar Ve cm-mol- mass TJK P/bar Ze T/K Cyclohexane 84.161 0.210 553.6 40.73 0.273 308. 353.9 Calculate the molar volume of cyclohexane vapor by using: (a) Ideal gas equation of state. [4 marks] [8 marks) (b) Lee-Kesler equation of state. [8 marks) (c) 2 terms virial equation of state with generalized correlation for B.
Step by Step Solution
3.54 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


