Answered step by step
Verified Expert Solution
Question
1 Approved Answer
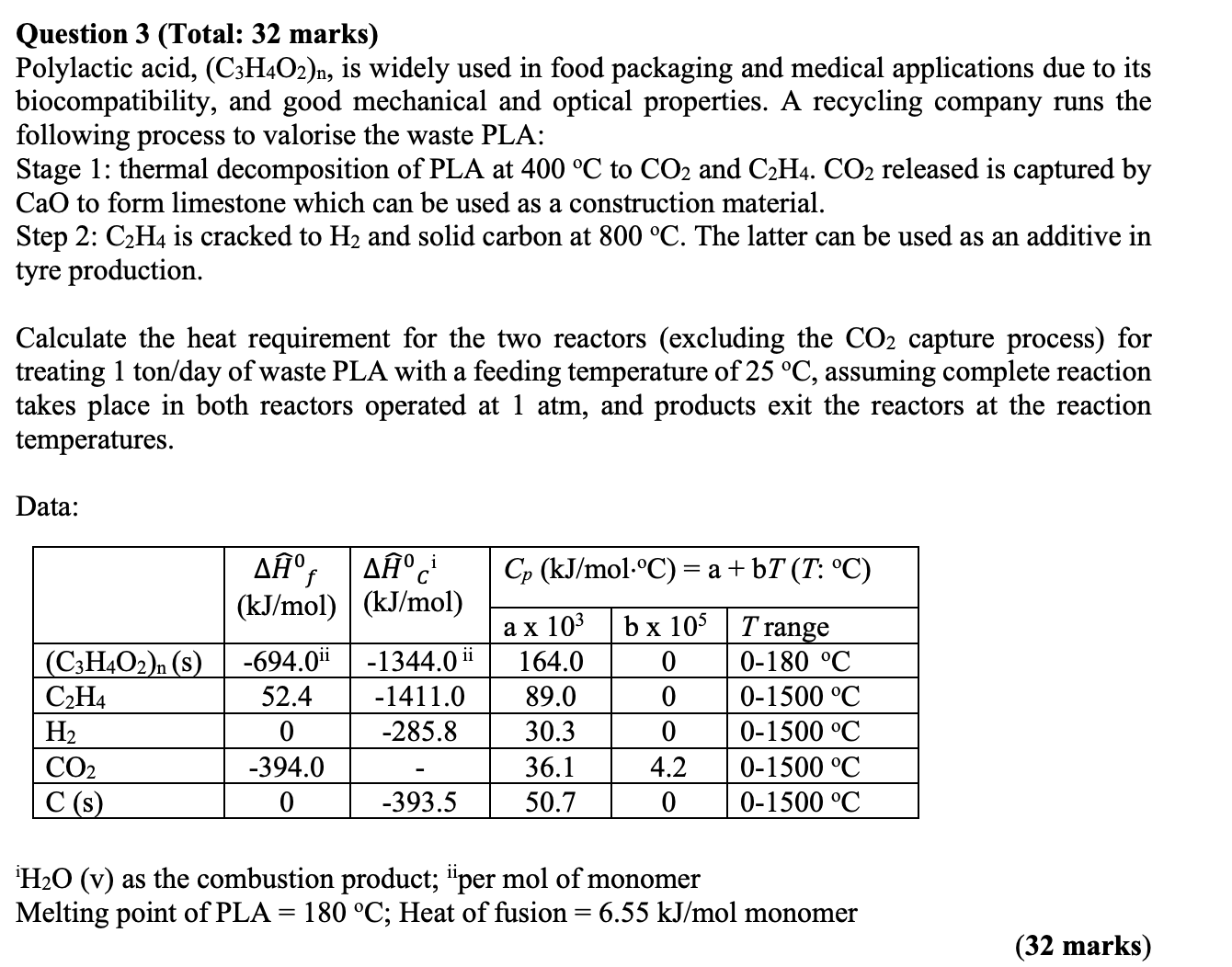
Question 3 ( Total: 3 2 marks ) Polylactic acid, ( C 3 H 4 O 2 ) n , is widely used in food
Question Total: marks
Polylactic acid, is widely used in food packaging and medical applications due to its
biocompatibility, and good mechanical and optical properties. A recycling company runs the
following process to valorise the waste PLA:
Stage : thermal decomposition of PLA at to and released is captured by
CaO to form limestone which can be used as a construction material.
Step : is cracked to and solid carbon at The latter can be used as an additive in
tyre production.
Calculate the heat requirement for the two reactors excluding the capture process for
treating tonday of waste PLA with a feeding temperature of assuming complete reaction
takes place in both reactors operated at atm, and products exit the reactors at the reaction
temperatures.
Data:
v as the combustion product; mol of monomer
Melting point of PLA ; Heat of fusion monomer
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


