Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 6 A heat engine is operated using the Carnot cycle shown. A Carnot cycle has two isothermal processes B and C, and two
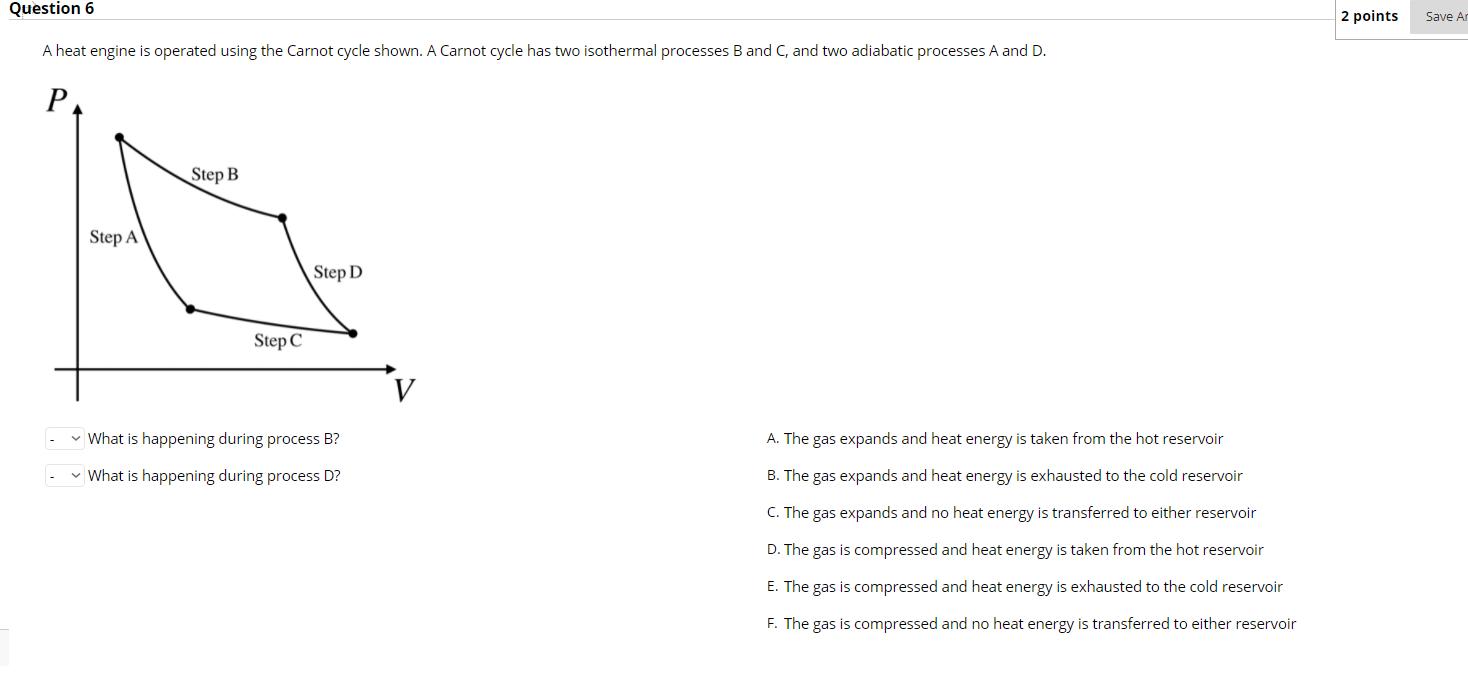




Question 6 A heat engine is operated using the Carnot cycle shown. A Carnot cycle has two isothermal processes B and C, and two adiabatic processes A and D. P Step A Step B Step C Step D What is happening during process B? What is happening during process D? A. The gas expands and heat energy is taken from the hot reservoir B. The gas expands and heat energy is exhausted to the cold reservoir C. The gas expands and no heat energy is transferred to either reservoir D. The gas is compressed and heat energy is taken from the hot reservoir E. The gas is compressed and heat energy is exhausted to the cold reservoir F. The gas is compressed and no heat energy is transferred to either reservoir 2 points Save An Question 8 Which of the following statements best describes what happens to the hot reservoir for a heat pump during one cycle? O Heat energy flows from the hot reservoir to the engine by an amount greater than the work done on the engine O Heat energy flows from the hot reservoir to the engine by an amount less than the work done on the engine Heat energy flows from the engine to the hot reservoir by an amount greater than the work done on the engine O Heat energy flows from the engine to the hot reservoir by an amount less than the work done on the engine 2 points Save Answer Question 9 Answer the following questions concerning the entropy change for heat engines, heat pumps, or refrigerators. For each cycle of a heat engine, the entropy of the hot reservoir... For each cycle of a heat pump, the entropy of the cold reservoir... For each cycle of a refrigerator, the entropy of the hot reservoir... - For each cycle of a carnot engine, the entropy of everything (hot reservoir + cold reservoir + engine)... . A. increases B. decreases C. stays the same 2 points S A box cycle is shown for a refridgerator. Which diagram correctly shows how heat energy is transferred during this cycle? An arrow pointing inside the cycle represents heat energy transferred to the gas, while an arrow pointing out of the cycle represents heat energy being transferred out of the gas. P P- P- P- P- P- P- Step 4 2 Q Step 3 Step 1 2 V Q3 Q Step 2 V 2 l V V O P- P P P- P- O P P- P- Q4 V Q4 2 2 2 Q3 V V
Step by Step Solution
★★★★★
3.39 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
P Q8 8 Adhabahc compression 13 B Isothermal T2 Ti d ex Q1 to pansion b Duri...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


