Answered step by step
Verified Expert Solution
Question
1 Approved Answer
question 6 and 8 6. Based on the density, refer to Table 1: Densities of Selected Metals and Identify your unknown solid. Unknown Solid: Be
question 6 and 8 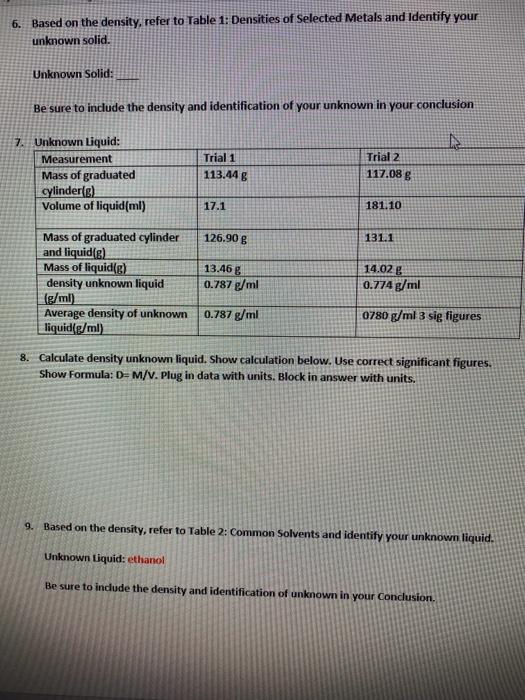
6. Based on the density, refer to Table 1: Densities of Selected Metals and Identify your unknown solid. Unknown Solid: Be sure to include the density and identification of your unknown in your conclusion 7. Unknown Liquid: Measurement Mass of graduated cylinder(e) Volume of liquid(ml) Trial 1 113.44g Trial 2 117.08 g 17.1 181.10 Mass of graduated cylinder 126.90 g 131.1 and liquid(g) Mass of liquid[g) 13.46 g 14.02 g density unknown liquid 0.787 g/ml 0.774 g/ml (g/ml) Average density of unknown 0.787 g/ml 0780 g/ml 3 sig figures | liquid/g/ml) 8. Calculate density unknown liquid. Show calculation below. Use correct significant figures. Show Formula: D= M/V. Plug in data with units. Block in answer with units. 9. Based on the density, refer to Table 2: Common Solvents and identify your unknown liquid. Unknown Liquid: ethanol Be sure to include the density and identification of unknown in your conclusion. 6. Based on the density, refer to Table 1: Densities of Selected Metals and Identify your unknown solid. Unknown Solid: Be sure to include the density and identification of your unknown in your conclusion 7. Unknown Liquid: Measurement Mass of graduated cylinder(e) Volume of liquid(ml) Trial 1 113.44g Trial 2 117.08 g 17.1 181.10 Mass of graduated cylinder 126.90 g 131.1 and liquid(g) Mass of liquid[g) 13.46 g 14.02 g density unknown liquid 0.787 g/ml 0.774 g/ml (g/ml) Average density of unknown 0.787 g/ml 0780 g/ml 3 sig figures | liquid/g/ml) 8. Calculate density unknown liquid. Show calculation below. Use correct significant figures. Show Formula: D= M/V. Plug in data with units. Block in answer with units. 9. Based on the density, refer to Table 2: Common Solvents and identify your unknown liquid. Unknown Liquid: ethanol Be sure to include the density and identification of unknown in your conclusion 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


