Answered step by step
Verified Expert Solution
Question
1 Approved Answer
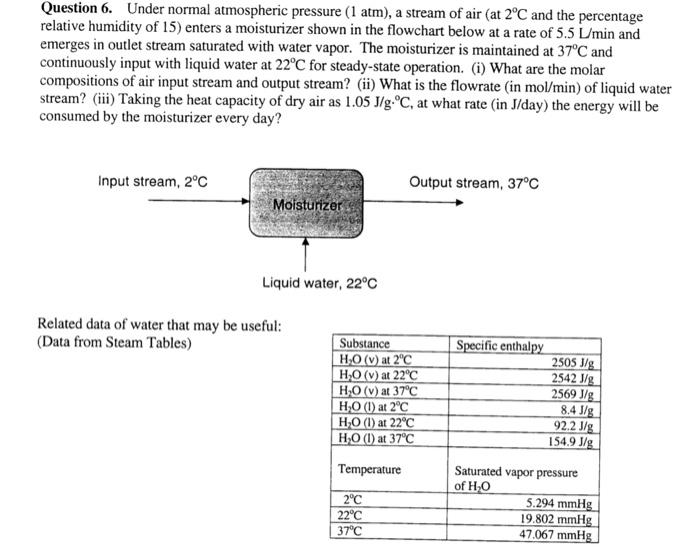
Question 6. Under normal atmospheric pressure (1 atm), a stream of air (at 2C and the percentage relative humidity of 15) enters a moisturizer shown
Question 6. Under normal atmospheric pressure (1 atm), a stream of air (at 2C and the percentage relative humidity of 15) enters a moisturizer shown in the flowchart below at a rate of 5.5 L/min and emerges in outlet stream saturated with water vapor. The moisturizer is maintained at 37C and continuously input with liquid water at 22C for steady-state operation. (i) What are the molar compositions of air input stream and output stream? (ii) What is the flowrate (in mol/min) of liquid water stream? (iii) Taking the heat capacity of dry air as 1.05 J/g C, at what rate (in J/day) the energy will be consumed by the moisturizer every day? Input stream, 2C Moisturizer Liquid water, 22C Related data of water that may be useful: (Data from Steam Tables) Substance HO (v) at 2C HO (v) at 22C HO (v) at 37C HO (1) at 2C HO (1) at 22C HO (1) at 37C Temperature Output stream, 37C 2C 22C 37C Specific enthalpy 2505 J/g 2542 J/g 2569 J/g 8.4 J/g 92.2 J/g 154.9 J/g Saturated vapor pressure of HO 5.294 mmHg 19.802 mmHg 47.067 mmHg
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


