Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question required to be answered: 'For Practice 13.7' with step-by-step solution. ANS: 2.09*10^-5 L/mol s Thank you. SOLUTION To find the frequency factor and activation
Question required to be answered: 'For Practice 13.7' with step-by-step solution. ANS: 2.09*10^-5 L/mol s
Thank you.
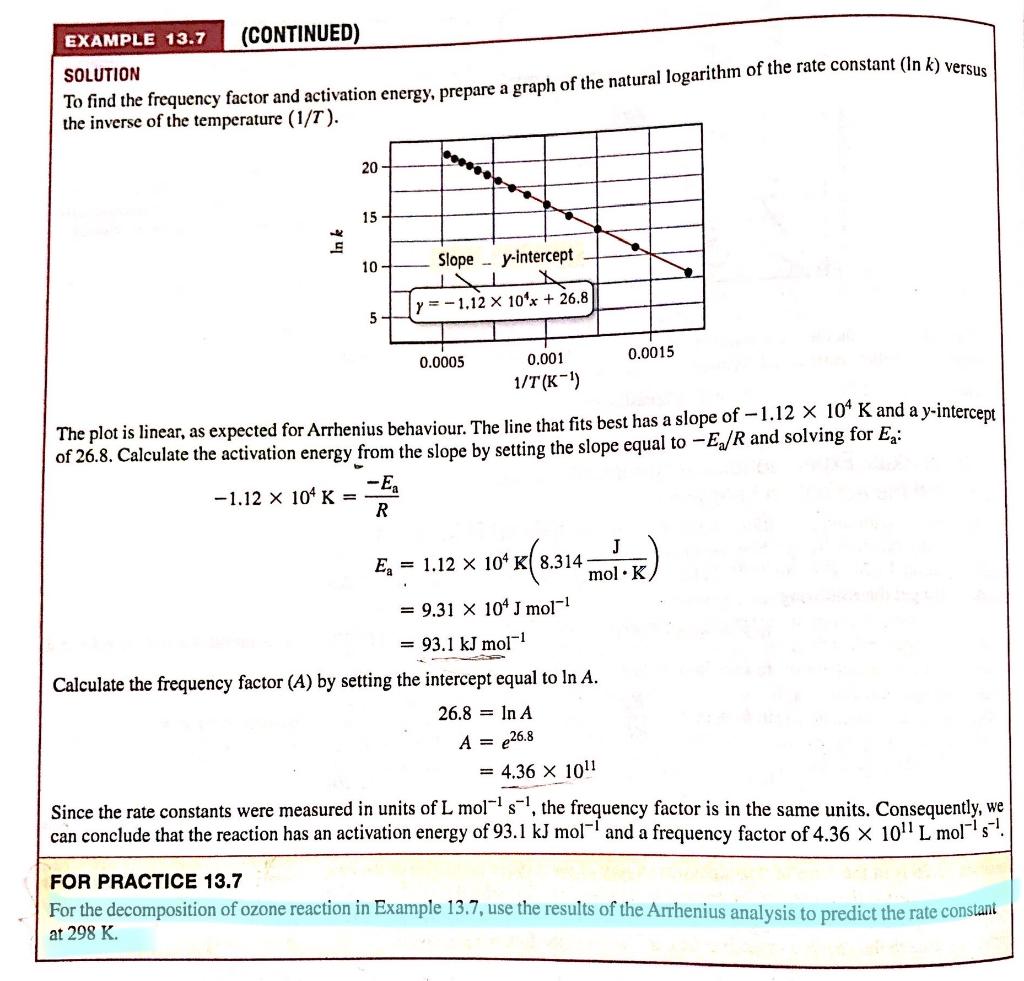
SOLUTION To find the frequency factor and activation energy, prepare a graph of the natural logarithm of the rate constant (ln k ) versus the inverse of the temperature (1/T). The plot is linear, as expected for Arrhenius behaviour. The line that fits best has a slope of 1.12104K and a y-intercept of 26.8. Calculate the activation energy from the slope by setting the slope equal to Ea/R and solving for Ea : 1.12104K=REaEa=1.12104K(8.314molKJ)=9.31104Jmol1=93.1kJmol1 Calculate the frequency factor (A) by setting the intercept equal to lnA. 26.8A=lnA=e26.8=4.361011 Since the rate constants were measured in units of Lmol1s1, the frequency factor is in the same units. Consequently, we can conclude that the reaction has an activation energy of 93.1kJmol1 and a frequency factor of 4.361011Lmol1s1. FOR PRACTICE 13.7 For the decomposition of ozone reaction in Example 13.7, use the results of the Arrhenius analysis to predict the rate constant at 298KStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


