Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Questions are at the end. Please help! Understanding the Experiment In this experiment, you will oxidize a secondary alcohol, isoborneol, to a ketone, camphor. Secondary
Questions are at the end. Please help! 


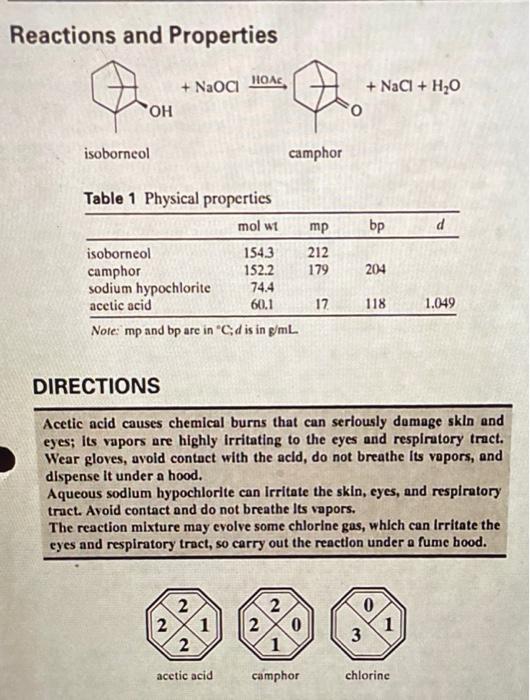



Understanding the Experiment In this experiment, you will oxidize a secondary alcohol, isoborneol, to a ketone, camphor. Secondary alcohols can be converted to ketones by pow- erful oxidizing agents such as chromic acid, but many chromium compounds are highly toxic and corrosive, and some are known to cause cancer. They also present a difficult disposal problem because they cannot legally be discharged into waterways or other places where they might harm the envi- ronment. For these reasons, you will use a safer and more environmentally friendly oxidizing agent, the familiar household laundry bleach that is sold under such trade names as Clorox and Javex. Most chlorine bleaches contain either 5.25% or 6.0% sodium hypochlorite (NaOCI) in an aqueous solu- tion. Adding a little acetic acid facilitates oxidation by converting sodium hypochlorite to hypochlorous acid (HOCI), which is probably the active oxidizing agent. Because sodium hypochlorite solutions evolve some chlorine gas, the reaction is carried out under a fume hood. In some previous experiments, you may have heated the reaction mix- ture to speed up the reaction. In this experiment, you will need to slow it down instead, because the oxidation of isoborneol is exothermic and the heat evolved can lead to the formation of unwanted by-products such as camphoric acid. You will control the reaction rate by adding the sodium 2 A Green Synthesis of Camphor COOH COOH camphoric acid hypochlorite, a little at a time, from a separatory-addition funnel rather than combining all of the reactants at once. You will also use a cooling bath as necessary to keep down the temperature in the reaction Nask, and monitor the temperature with a thermometer as described in OP-9. When a reaction takes place under reflux, the boiling action helps mix the reactants. In this experiment, the reactants are mixed using a magnetic stirrer or by shaking and swirling the flask after each addition. To ensure a complete reaction, you must add enough sodium hypochlorite solution to keep the oxidizing agent in excess throughout the reaction. Because the NaOCI concentrations of different chlorine bleaches may differ and will decrease with age, you can't be sure that the amount of bleach that you add at first will be enough, so you will have to test the reaction mixture peri- odically to see whether there is still excess oxidant present. When HOCI is present in excess, a drop of the acidic reaction mixture placed on an indicator paper impregnated with starch and potassium iodide will oxidize iodide ions to iodine, which turns the starch a deep blue-black color. The color near the center of the drop may be bleached white, but some color should remain around its edges. Any excess HOCI that remains after the reaction is over can be destroyed by treatment with the reducing agent sodium bisulfite, according to the following equation: HOCI + HSO, HCI + HSO, The melting point of a solid equals the freezing point of the corresponding liquid. Because of its compact molecular structure, camphor changes directly from a solid to a vapor when heated, which allows it to be purified by subli- mation. Isoborneol also sublimes at elevated temperatures, so the sublimed camphor will probably contain some unreacted isoborncol. Assuming that isoborneol is the only significant impurity in the product, its purity can be estimated with good accuracy from its melting point, because camphor has an unusually large freezing-point depression constant. The product can be regarded as a solid solution with camphor as the solvent and isoborncol as the solute, so you can use the following equation to calculate the molal concentration (m) of isoborneol in the product: AT = K, Xm AT = melting point depression (reported mp - observed mp) K, = freezing-point depression constant for camphor = 40C kg mol"! m = molal concentration of isoborneol (mol isoborneol/kg camphor) Knowing m, the number of moles of isoborneol per kilogram of camphor you can calculate the mass percent of isoborneol and camphor in the product. This experiment has several green features in addition to the use of a more benign oxidizing agent. Isoborneol is manufactured from a com- ponent of turpentine (a renewable resource), the synthesis uses no sol- vents other than the water present in the laundry bleach, and the only by-products are water and sodium chloride. Because sodium hypochlo- rite is used widely to disinfect water, it is often present in municipal drinking water, and the EPA has concluded that such uses present no unreasonable adverse effects to the environment. It is toxic to freshwater fish and invertebrates, however, so its release into the environment should be avoided. Reactions and Properties + NaOg HOA + NaCl + H2O o isoborneol camphor bp d Table 1 Physical properties mol wt isoborneol 1543 camphor 152.2 sodium hypochlorite 74.4 acetic acid 60.1 Note: mp and bp arc in "cd is in g/mL mp 212 179 204 17 118 1.049 DIRECTIONS Acetic acid causes chemical burns that can seriously domage skin and eyes; Its vapors are highly irritating to the eyes and respiratory tract. Wear gloves, avoid contact with the acid, do not breathe Its vapors, and dispense It under a hood. Aqueous sodlum hypochlorite can Irritate the skin, eyes, and respiratory tract. Avoid contact and do not breathe Its vapors. The reaction mixture may evolve some chlorine gas, which can Irritate the eyes and respiratory tract, so carry out the reaction under a fume bood. 0 2 2 2 1 2 2 0 1 3 acetic acid camphor chlorine Reaction. Under the hood, combine 25.0 mmol of isoborncol with 2.0 mL of glacial acetic acid in a 125-mL Erlenmeyer flask. Mix in 5.0 mL of 5.25% (or 4.4 mL of 6.0%) sodium hypochlorite solution (Clorox or another hypochlorite laundry bleach). Measure another 40 mL of 5.25% (or 35 mL of 6.0%) sodium hypochlorite solution into a separatory-addition funnel, stopper the funnel, and support it over the fask. Add [OP-11] the NaOCI solution to the reaction mixture in small portions, with vigorous swirling or magnetic stirring (OP-10), for a period of 10 minutes or more. Use a ther- mometer to monitor the temperature (OP-9) of the reaction mixture, and control the rate of addition so that the temperature remains below 50C. A Green Synthesis of Camphor Have an ice/water bath handy to cool (OP-8) the reaction mixture if its temperature reaches 50C. When the addition is complete, seal the flask with Parafilm and stir [OP-10) the reactants (or swirl the flask frequently) at room temperature for 30 minutes or more. Every 5 minutes or so, test the reaction mixture for excess hypochlorite by transferring a drop of the solution to a strip of starch-iodide paper. If at any time the test is negative, add enough sodium hypochlorite solution (about 1 mL at a time) to the reaction mixture to give a positive test. When the reaction period is over, again test the reaction mix- ture with starch-iodide paper. If the test is positive, add enough saturated sodium bisulfite solution dropwise to give a negative test. Separation. Cool [OP-8) the reaction mixture to 5C or below in an ice/water bath. Collect the product by vacuum filtration (OP-16), washing it on the filter [OP-26a) with two portions of ice-cold water. Dry (OP-26b) the crude product at room temperature, not in an oven. At your instructor's request, weigh the crude product and save a small amount of it for a melting point measurement. Purification and Analysis. Purify the crude product by sublimation (OP-29), taking care not to char the solid by overheating. Weigh [OP-4) the sublimate, dry (OP-26b) it at room temperature (if necessary), and measure its melting point (OP-33). Taking as its melting point the temperature at which the solid was completely liquefied, calculate the mass percentages of isoborneol and camphor in your purified product. Include a calculation of percent yield in your report. Exercises 1. In this experiment, you started with a white, strong-smelling solid and ended up with a white, strong-smelling solid. What evidence leads you to conclude that these two solids are in fact different compounds and that you didn't just isolate the unreacted starting material? 2. Following the format in the "Planning an Experiment" appendix, con- struct a flow diagram for the synthesis of camphor. 3. The equation in the "Reactions and Properties" section shows only one isoborneol enantiomer and the camphor enantiomer it forms. Find out from your instructor if the isoborneol you used was the right-handed (R) enantiomer shown, the left-handed (S) enantiomer, or an equimo- lar mixture of both. Then rewrite the equation showing the correct stereochemistry of the reactants and products 4. (a) Calculate the atom economy and reaction efficiency of this synthesis (b) Describe some green features of your synthesis, and any that aren't so green. 5. Describe how each of the following experimental errors or variations might affect your results (a) You omitted the 30-minute reaction period after the addition step. (b) You added the sodium hypochlorite solution all at once. (c) You mistook a negative starch-iodide test for a positive one and stopped adding sodium hypochlorite solution midway through the reaction period 






Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


