Answered step by step
Verified Expert Solution
Question
1 Approved Answer
reaction engineering please show all steps 1. Below is a Thermodynamic equilibrium calculated for Dry reforming of ethane reaction. The line is the temperature at
reaction engineering please show all steps
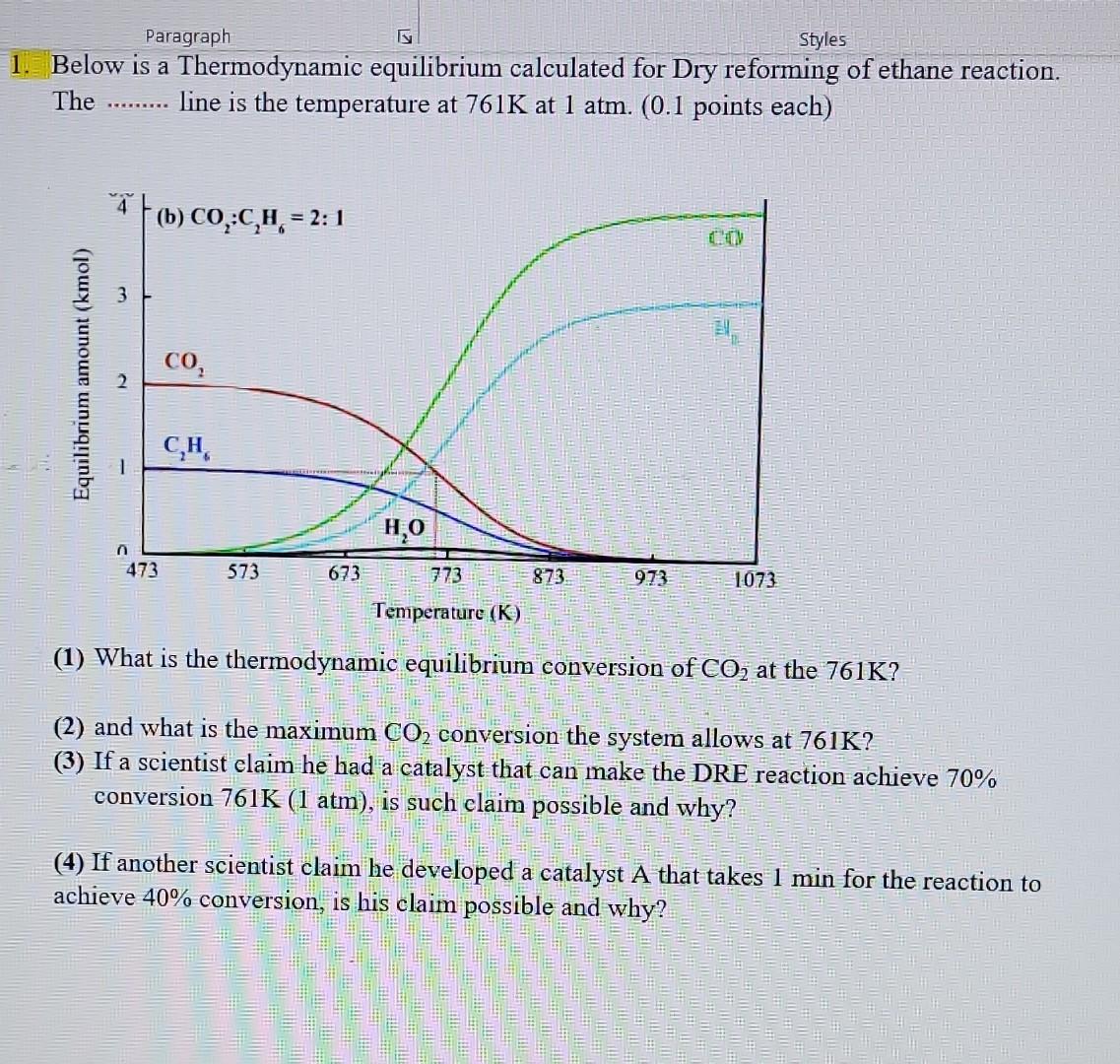
1. Below is a Thermodynamic equilibrium calculated for Dry reforming of ethane reaction. The line is the temperature at 761K at 1atm. (0.1 points each) (1) What is the thermodynamic equilibrium conversion of CO2 at the 761K ? (2) and what is the maximum CO2 conversion the system allows at 761K ? (3) If a scientist elaim he had a catalyst that can make the DRE reaction achieve 70% conversion 761K(1atm), is such claim possible and why? (4) If another scientist claim he developed a catalyst A that takes 1 min for the reaction to achieve 40% conversion, is his claim possible and why? 1. Below is a Thermodynamic equilibrium calculated for Dry reforming of ethane reaction. The line is the temperature at 761K at 1atm. (0.1 points each) (1) What is the thermodynamic equilibrium conversion of CO2 at the 761K ? (2) and what is the maximum CO2 conversion the system allows at 761K ? (3) If a scientist elaim he had a catalyst that can make the DRE reaction achieve 70% conversion 761K(1atm), is such claim possible and why? (4) If another scientist claim he developed a catalyst A that takes 1 min for the reaction to achieve 40% conversion, is his claim possible and why
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


