Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Read the attached case, The Panalba Decision. As a member of the board, you must help to reach a decision at today's meeting. The Chairperson
Read the attached case, "The Panalba Decision." As a member of the board, you must help to reach a decision at today's meeting. The Chairperson of the Board, Dr. UpJohn, has provided the case background information to each board member. Your task is to select one of the five alternatives from the "Panalba Decision" for:
(1) The U.S. market; and (2) assuming the FDA does ultimately ban Panalba in the U.S., the foreign market (which will not be affected by the ban).
Inside Director: Chief Financial Officer money, money, money, is your concern. You will base your decision upon what will be most profitable for the company. You are concerned with both short term and long term profits for shareholders.

has to be atleast 400 worde
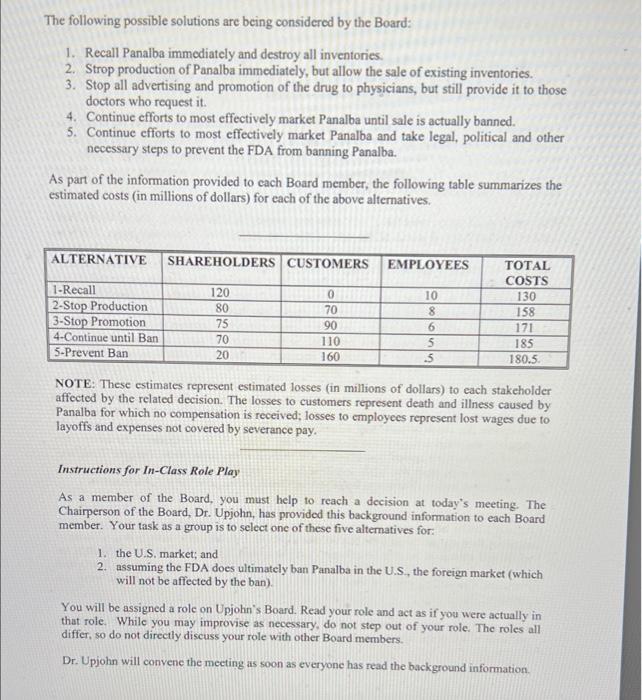
THE PANALBA DECISION Background You are a member of the Board of Directors of the Upjohn Corporation. The company has called a Special Meeting of the Board to provide some guidance as to what should be done with one of the company's products, a drug named "Panalba." Panalba is a fixed-ratio (containing a combination of drugs) antibiotic drug sold by prescription It has been on the market for over 12 years and has been highly successful for the company. It now accounts for over 250 million dollars per year, which is 14 percent of Upjohn's gross profit in the United States. Profits from foreign markets, where Panalba is marketed under a different name, are roughly comparable to those in the U.S. Over the past 25 years, there have been numerous medical researchers (c.g., the AMA's Council on Drugs) that have objected to the sale of the vast majority of fixed-ratio drugs. The basic argument against these drugs is: (1) there is no evidence that fixed-ratio drugs provide improved benefits over single drugs, and (2) the possibility of detrimental side effects, including death, is at least doubled. These researchers, for example, have estimated that Panalba is contributing to approximately 22 to as many as 50 unnecessary deaths per year, deaths that could have been prevented if the patients had used a substitute, single drug made by one of Upjohn's competitors. Due to the difficulties of proving a definitive cause-effect relationship, however, Panalba (as well as other fixed-ratio drugs) have remained on the market and many doctors continue to use them. These drugs offer a type of shotgun approach for the doctor who is unsure of his or her diagnosis Recently, based on their review of the situation, a National Academy of Science-National Research Council Panel, a group of highly-respected, impartial scientists, recommended unanimously that the Food and Drug Administration (FDA) ban the sale of Panalba. One of the members of the panel was quoted in the press noting. "There are few instances in medicine when so many experts have agreed unanimously and without reservation (about banning Panalba)." These experts were clear that while any drug has the possibility of dangerous side effects, the costs associated with the fixed-ratio Panalba drug clearly outweighed the possible benefits. This Special Board Meeting was called due to an emergency situation. The FDA had just informed Upjohn of its intent to ban Panalba in the U.S. and wanted to give the company a chance for final appeal. Should the ban become effective, Upjohn would have to stop all sales of Panalba and attempt to remove inventories from the market. Upjohn has no close substitute for Panalba, so doctors would switch their patients to substitute drugs readily available from other companies. The selling price of these substitute drugs is roughly the same as the price for Panalba The Board's assumption is that it is unlikely that bad publicity from this case would have any significant effect on the sale of Upjohn's other products or its long-term profitability, The following possible solutions are being considered by the Board: 1. Recall Panalba immediately and destroy all inventories. 2. Strop production of Panalba immediately, but allow the sale of existing inventories. 3. Stop all advertising and promotion of the drug to physicians, but still provide it to those doctors who request it. 4. Continue efforts to most effectively market Panalba until sale is actually banned. 5. Continue efforts to most effectively market Panalba and take legal, political and other necessary steps to prevent the FDA from banning Panalba. As part of the information provided to cach Board member, the following table summarizes the estimated costs (in millions of dollars) for each of the above alternatives, ALTERNATIVE SHAREHOLDERS CUSTOMERS EMPLOYEES 1-Recall 2-Stop Production 3-Stop Promotion 4-Continue until Ban 5-Prevent Ban 120 80 75 70 20 0 70 90 110 160 uolo 10 8 6 5 s TOTAL COSTS 130 158 171 185 180.5 NOTE: These estimates represent estimated losses (in millions of dollars) to each stakeholder affected by the related decision. The losses to customers represent death and illness caused by Panalba for which no compensation is received; losses to employees represent lost wages due to layoffs and expenses not covered by severance pay. Instructions for In-Class Role Play As a member of the Board, you must help to reach a decision at today's meeting. The Chairperson of the Board, Dr. Upjohn, has provided this background information to each Board member. Your task as a group is to select one of these five alternatives for: 1. the U.S. market; and 2. assuming the FDA does ultimately ban Panalba in the U.S., the foreign market (which will not be affected by the ban) You will be assigned a role on Upjohn's Board. Read your role and act as if you were actually in that role. While you may improvise as necessary, do not step out of your role. The roles all differ, so do not directly discuss your role with other Board members. Dr. Upjohn will convenc the meeting as soon as everyone has read the background information THE PANALBA DECISION Background You are a member of the Board of Directors of the Upjohn Corporation. The company has called a Special Meeting of the Board to provide some guidance as to what should be done with one of the company's products, a drug named "Panalba." Panalba is a fixed-ratio (containing a combination of drugs) antibiotic drug sold by prescription It has been on the market for over 12 years and has been highly successful for the company. It now accounts for over 250 million dollars per year, which is 14 percent of Upjohn's gross profit in the United States. Profits from foreign markets, where Panalba is marketed under a different name, are roughly comparable to those in the U.S. Over the past 25 years, there have been numerous medical researchers (c.g., the AMA's Council on Drugs) that have objected to the sale of the vast majority of fixed-ratio drugs. The basic argument against these drugs is: (1) there is no evidence that fixed-ratio drugs provide improved benefits over single drugs, and (2) the possibility of detrimental side effects, including death, is at least doubled. These researchers, for example, have estimated that Panalba is contributing to approximately 22 to as many as 50 unnecessary deaths per year, deaths that could have been prevented if the patients had used a substitute, single drug made by one of Upjohn's competitors. Due to the difficulties of proving a definitive cause-effect relationship, however, Panalba (as well as other fixed-ratio drugs) have remained on the market and many doctors continue to use them. These drugs offer a type of shotgun approach for the doctor who is unsure of his or her diagnosis Recently, based on their review of the situation, a National Academy of Science-National Research Council Panel, a group of highly-respected, impartial scientists, recommended unanimously that the Food and Drug Administration (FDA) ban the sale of Panalba. One of the members of the panel was quoted in the press noting. "There are few instances in medicine when so many experts have agreed unanimously and without reservation (about banning Panalba)." These experts were clear that while any drug has the possibility of dangerous side effects, the costs associated with the fixed-ratio Panalba drug clearly outweighed the possible benefits. This Special Board Meeting was called due to an emergency situation. The FDA had just informed Upjohn of its intent to ban Panalba in the U.S. and wanted to give the company a chance for final appeal. Should the ban become effective, Upjohn would have to stop all sales of Panalba and attempt to remove inventories from the market. Upjohn has no close substitute for Panalba, so doctors would switch their patients to substitute drugs readily available from other companies. The selling price of these substitute drugs is roughly the same as the price for Panalba The Board's assumption is that it is unlikely that bad publicity from this case would have any significant effect on the sale of Upjohn's other products or its long-term profitability, The following possible solutions are being considered by the Board: 1. Recall Panalba immediately and destroy all inventories. 2. Strop production of Panalba immediately, but allow the sale of existing inventories. 3. Stop all advertising and promotion of the drug to physicians, but still provide it to those doctors who request it. 4. Continue efforts to most effectively market Panalba until sale is actually banned. 5. Continue efforts to most effectively market Panalba and take legal, political and other necessary steps to prevent the FDA from banning Panalba. As part of the information provided to cach Board member, the following table summarizes the estimated costs (in millions of dollars) for each of the above alternatives, ALTERNATIVE SHAREHOLDERS CUSTOMERS EMPLOYEES 1-Recall 2-Stop Production 3-Stop Promotion 4-Continue until Ban 5-Prevent Ban 120 80 75 70 20 0 70 90 110 160 uolo 10 8 6 5 s TOTAL COSTS 130 158 171 185 180.5 NOTE: These estimates represent estimated losses (in millions of dollars) to each stakeholder affected by the related decision. The losses to customers represent death and illness caused by Panalba for which no compensation is received; losses to employees represent lost wages due to layoffs and expenses not covered by severance pay. Instructions for In-Class Role Play As a member of the Board, you must help to reach a decision at today's meeting. The Chairperson of the Board, Dr. Upjohn, has provided this background information to each Board member. Your task as a group is to select one of these five alternatives for: 1. the U.S. market; and 2. assuming the FDA does ultimately ban Panalba in the U.S., the foreign market (which will not be affected by the ban) You will be assigned a role on Upjohn's Board. Read your role and act as if you were actually in that role. While you may improvise as necessary, do not step out of your role. The roles all differ, so do not directly discuss your role with other Board members. Dr. Upjohn will convenc the meeting as soon as everyone has read the background information Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


