Question
(a) Explain why the reaction of SF4 with BF3 yields [SF3], whereas the reaction with CsF gives Cs[SFs]. (b) Suggest how SF4 might react
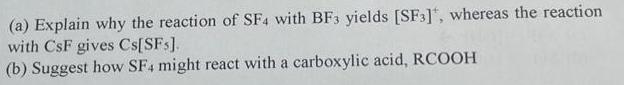
(a) Explain why the reaction of SF4 with BF3 yields [SF3], whereas the reaction with CsF gives Cs[SFs]. (b) Suggest how SF4 might react with a carboxylic acid, RCOOH
Step by Step Solution
3.46 Rating (172 Votes )
There are 3 Steps involved in it
Step: 1
1 SFs Bf3 SFY CSF to its lay This is because Brs is dedtron deficient in nature act as Lewis Ac...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Managerial Economics and Strategy
Authors: Jeffrey M. Perloff, James A. Brander
1st edition
978-0137036059, 133379094, 321566440, 137036051, 9780133379099, 978-0321566447
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



