Question
Recall the translational partition function in three dimensions: V/A, where A=h(B/2m)0.5. 10. What is the average translational energy in terms of and constants? 11.
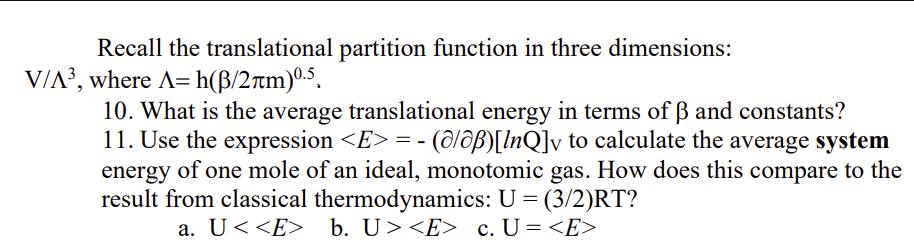
Recall the translational partition function in three dimensions: V/A, where A=h(B/2m)0.5. 10. What is the average translational energy in terms of and constants? 11. Use the expression = (alap)[InQ]v to calculate the average system energy of one mole of an ideal, monotomic gas. How does this compare to the result from classical thermodynamics: U = (3/2)RT? a. U < b. U> c. U=
Step by Step Solution
3.36 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
SOLUTION 10 The translational partition function in three dimensions can be written as VA 2mh36 where m is the mass of a particle is the inverse tempe...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physical Chemistry
Authors: Thomas Engel, Philip Reid
3rd edition
805338423, 080533842X, 978-0321812001
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



