Question
Redox reactions are also important to consider when studying the behavior of uranium in the environment. Uranium mill tailings often release UO 2 (aq) 2+
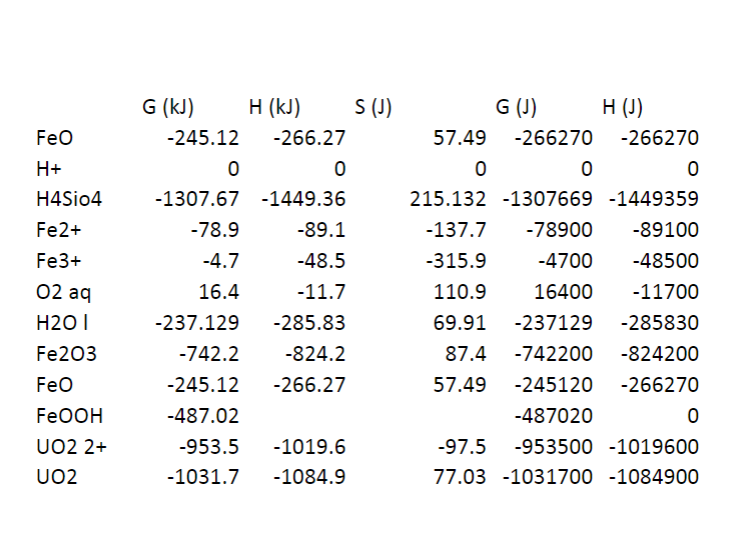
Redox reactions are also important to consider when studying the behavior of uranium in the environment. Uranium mill tailings often release UO2(aq)2+ to local streams. Consider the following reaction: Gf FeOOH= -490.6 kJ/mol FeO(wustite) + UO2(aq)2+ FeOOH(goethite) + UO2(uraninite) This is not balanced- you will balance this by adding H+, H2O, and/or O2 (aq) by the end of the problem.
Part A. What is the standard voltage (E) for the reduction cell reaction?
Part B. Calculate the equilibrium constant (Keq) for this reaction. Based on this calculation, what is the predicted activity of UO22+(aq) at pH = 5 at equilibrium?
Part C. Based on the equilibrium constant, what would you expect to happen if you added wustite to a stream contaminated with UO22+(aq) ? Would this be an effective way to prevent UO22+ (aq) from being transported further downstream? Why or why not?
Feo H+ H4Sio4 Fe2+ Fe3+ 02 aq G (kJ) H (kJ) S (1) -245.12 -266.27 0 0 -1307.67 -1449.36 -78.9 -89.1 -4.7 -48.5 16.4 -11.7 -237.129 -285.83 -742.2 -824.2 -245.12 -266.27 -487.02 -953.5 -1019.6 -1031.7 -1084.9 G (1) H() 57.49 -266270 -266270 0 0 0 215.132 -1307669 -1449359 -137.7 -78900 -89100 -315.9 -4700 -48500 110.9 16400 -11700 69.91 -237129 -285830 87.4 -742200 -824200 57.49 -245120 -266270 -487020 0 -97.5 -953500 -1019600 77.03 -1031700 -1084900 H201 Fe203 Feo FeOOH UO2 2+ UO2Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


