Answered step by step
Verified Expert Solution
Question
1 Approved Answer
References Mailings Review View Tell me Part D: Comparing Antacids Have you ever taken an antacid for relief heartburn? Stomach acid is approximately 0 .
References
Mailings
Review
View
Tell me
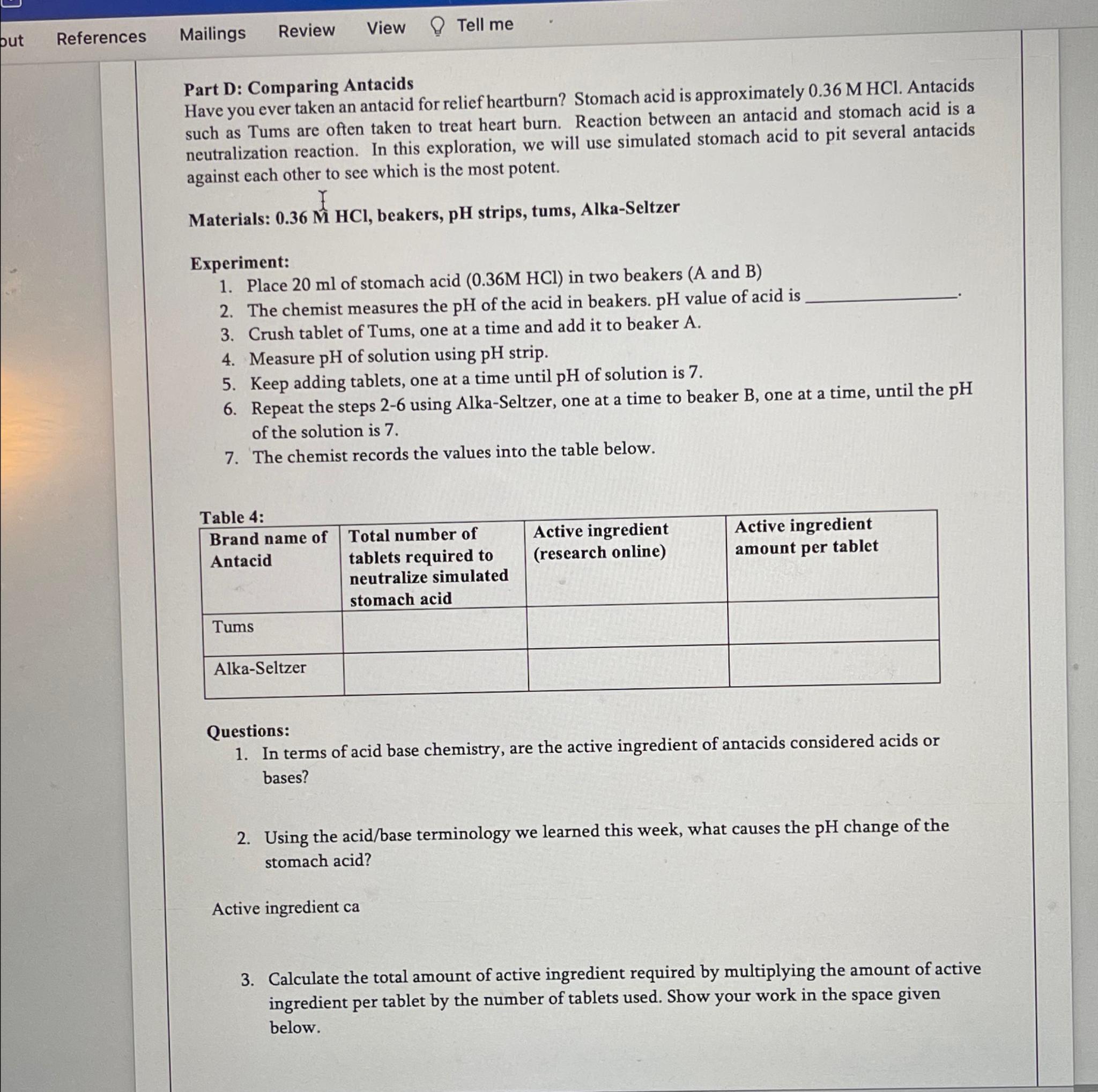
Part D: Comparing Antacids
Have you ever taken an antacid for relief heartburn? Stomach acid is approximately Antacids such as Tums are often taken to treat heart burn. Reaction between an antacid and stomach acid is a neutralization reaction. In this exploration, we will use simulated stomach acid to pit several antacids against each other to see which is the most potent.
Materials: beakers, strips, tums, AlkaSeltzer
Experiment:
Place of stomach acid in two beakers and
The chemist measures the of the acid in beakers. value of acid is
Crush tablet of Tums, one at a time and add it to beaker A
Measure of solution using strip.
Keep adding tablets, one at a time until of solution is
Repeat the steps using AlkaSeltzer, one at a time to beaker one at a time, until the of the solution is
The chemist records the values into the table below.
Table :
tabletableBrand name ofAntacidtableTotal number oftablets required toneutralize simulatedstomach acidtableActive ingredientresearch onlinetableActive ingredientamount per tabletTumsAlkaSeltzer,,,
Questions:
In terms of acid base chemistry, are the active ingredient of antacids considered acids or bases?
Using the acidbase terminology we learned this week, what causes the change of the stomach acid?
Active ingredient ca
Calculate the total amount of active ingredient required by multiplying the amount of active ingredient per tablet by the number of tablets used. Show your work in the space given below.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


