Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Repeat the analysis of Problem 2.58 to estimate the cohesive energy for a three-dimensional metal, including the effects of spin. Problem 2.58 In a

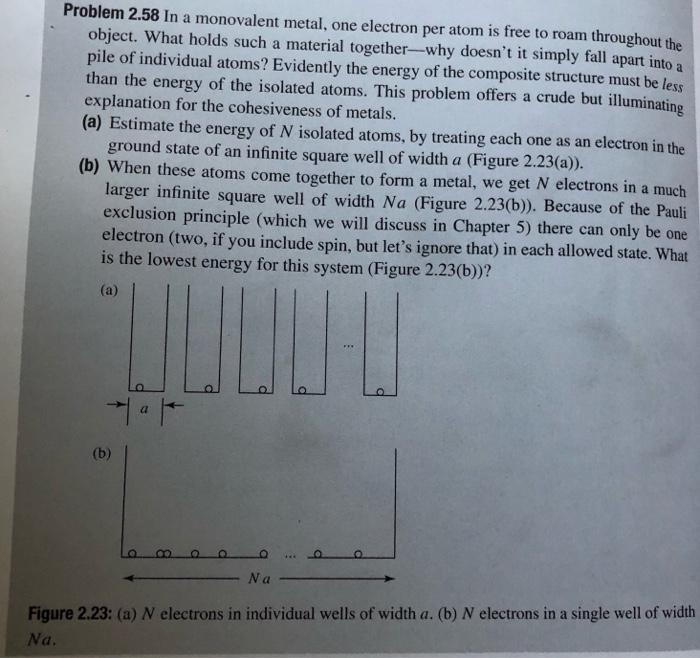

Repeat the analysis of Problem 2.58 to estimate the cohesive energy for a three-dimensional metal, including the effects of spin. Problem 2.58 In a monovalent metal, one electron per atom is free to roam throughout the object. What holds such a material together-why doesn't it simply fall apart into pile of individual atoms? Evidently the energy of the composite structure must be less than the energy of the isolated atoms. This problem offers a crude but illuminating explanation for the cohesiveness of metals. (a) Estimate the energy of N isolated atoms, by treating each one as an electron in the ground state of an infinite square well of width a (Figure 2.23(a)). (b) When these atoms come together to form a metal, we get N electrons in a much larger infinite square well of width Na (Figure 2.23(b)). Because of the Pauli exclusion principle (which we will discuss in Chapter 5) there can only be one electron (two, if you include spin, but let's ignore that) in each allowed state. What is the lowest energy for this system (Figure 2.23(b))? a (a) (b) 8, Na Figure 2.23: (a) N electrons in individual wells of width a. (b) N electrons in a single well of width Na. (c) The difference of these two energies is the cohesive energy of the metal-the energy it would take to tear it apart into isolated atoms. Find the cohesive energy per atom, in the limit of large N. (d) A typical atomic separation in a metal is a few Angstrm (say, a 4 A). What is the numerical value of the cohesive energy per atom, in this model? (Measured values are in the range of 2-4 e V.)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


