Question
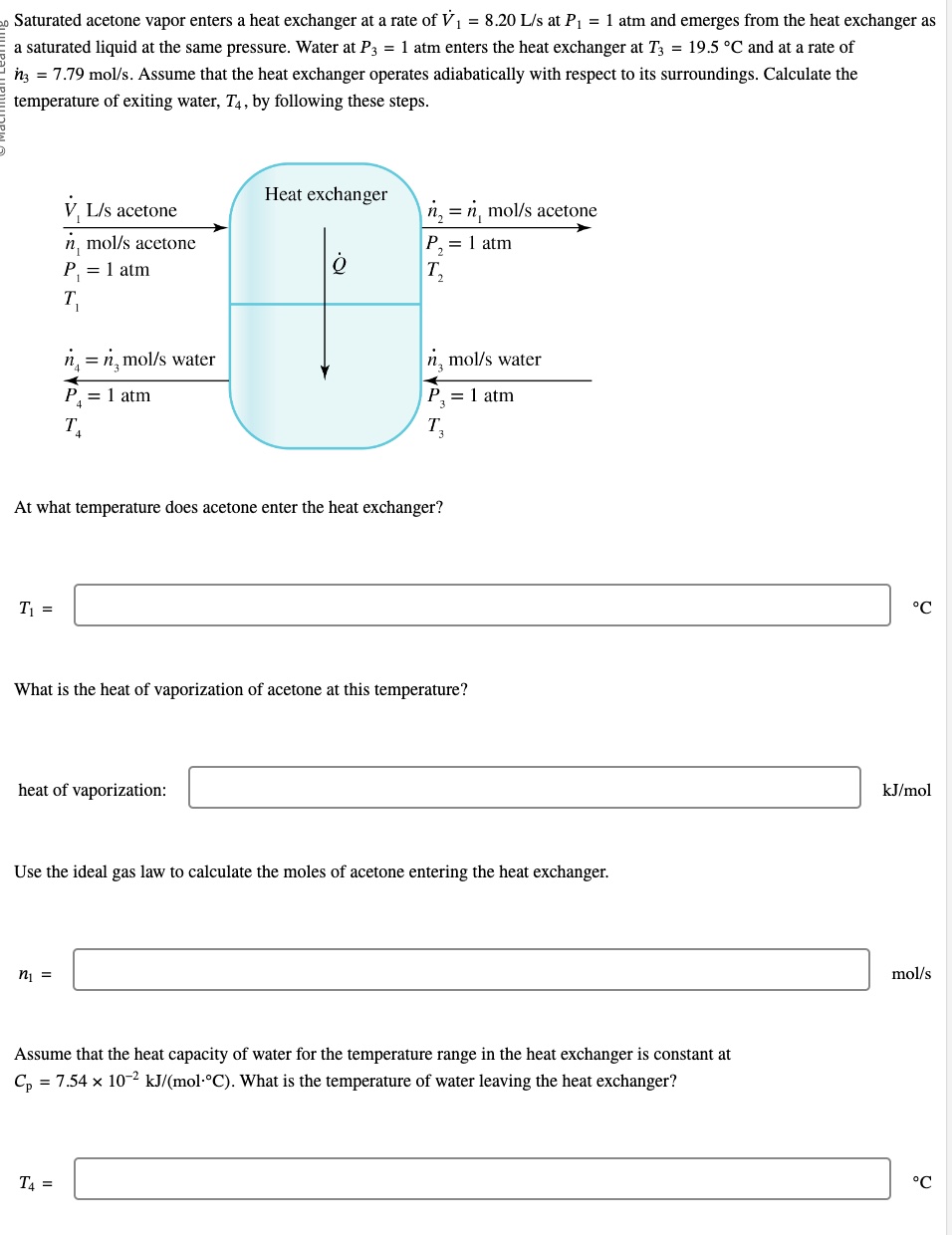
Saturated acetone vapor enters a heat exchanger at a rate of 1=8.20 L/s1=8.20 L/s at 1=1 atm1=1 atm and emerges from the heat exchanger as
Saturated acetone vapor enters a heat exchanger at a rate of 1=8.20 L/s1=8.20 L/s at 1=1 atm1=1 atm and emerges from the heat exchanger as a saturated liquid at the same pressure. Water at 3=1 atm3=1 atm enters the heat exchanger at 3=19.5 C3=19.5 C and at a rate of 3=7.79 mol/s.3=7.79 mol/s. Assume that the heat exchanger operates adiabatically with respect to its surroundings. Calculate the temperature of exiting water, 4,4, by following these steps.
1. At what temperature does acetone enter the heat exchanger? ______ *C
2. What is the heat of vaporization of acetone at this temperature? heat of vaporization: _____ kj/mol
3. Use the ideal gas law to calculate the moles of acetone entering the heat exchanger. 1=1= _______ mol/s
4. Assume that the heat capacity of water for the temperature range in the heat exchanger is constant at p=7.54102 kJ/(molC).p=7.54102 kJ/(molC). What is the temperature of water leaving the heat exchanger? 4=4= ____*C
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


