Question: Select all that apply. London forces Hydrogen Bonding Debye forces Metallic bonding Ionic bonding Covalent bonding Keesom forces A common medication for treating headaches is
Select all that apply.
London forces
Hydrogen Bonding
Debye forces
Metallic bonding
Ionic bonding
Covalent bonding
Keesom forces
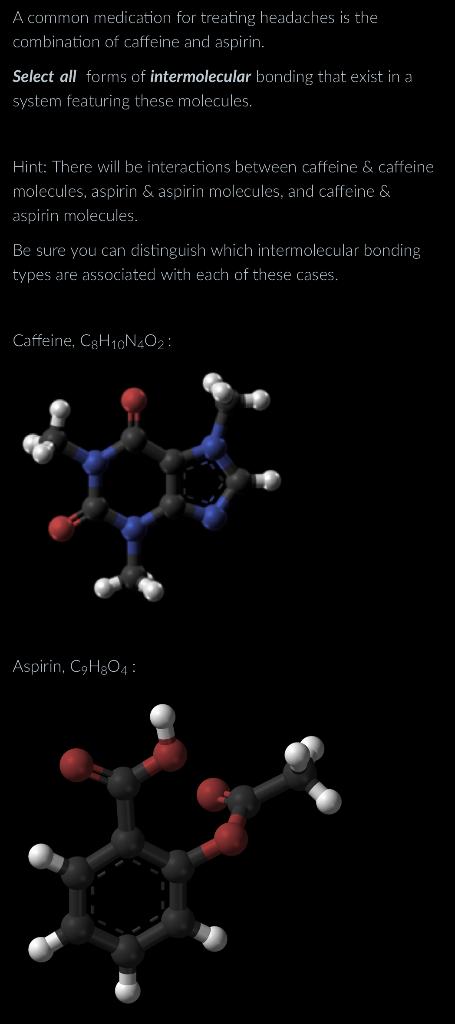
A common medication for treating headaches is the combination of caffeine and aspirin. Select all forms of intermolecular bonding that exist in a system featuring these molecules. Hint: There will be interactions between caffeine \& caffeine molecules, aspirin \& aspirin molecules, and caffeine & aspirin molecules. Be sure you can distinguish which intermolecular bonding types are associated with each of these cases. Caffeine, C8H10N4O2 : Aspirin, C9H8O4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


