Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Select the statements below that are TRUE. Explain why Benzene and its derivatives tend to undergo electrophilic aromatic addition reactions. Benzene and its derivatives tend
Select the statements below that are TRUE. Explain why
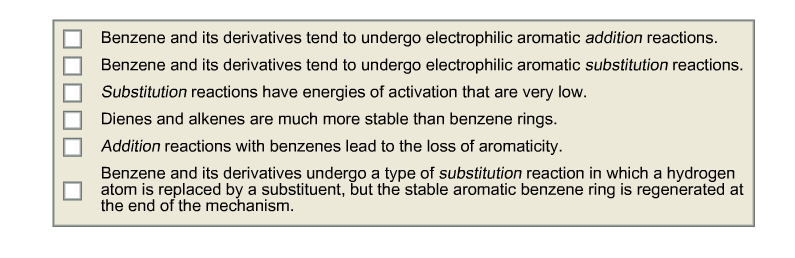
Benzene and its derivatives tend to undergo electrophilic aromatic addition reactions. Benzene and its derivatives tend to undergo electrophilic aromatic substitution reactions. Substitution reactions have energies of activation that are very low. Dienes and alkenes are much more stable than benzene rings. Addition reactions with benzenes lead to the loss of aromaticity. Benzene and its derivatives undergo a type of substitution reaction in which a hydrogen atom is replaced by a substituent, but the stable aromatic benzene ring is regenerated at the end of the mechanism.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
aBenzene and its derivatives tend to undergo electrophilic aromatic addition reactions bBenzene and its derivatives tend to undergo electrophilic arom...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


