Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Sensible heat is heat transfer that occurs when there are no and Latent heat is when a pure substance is liquefied or vaporized (phase
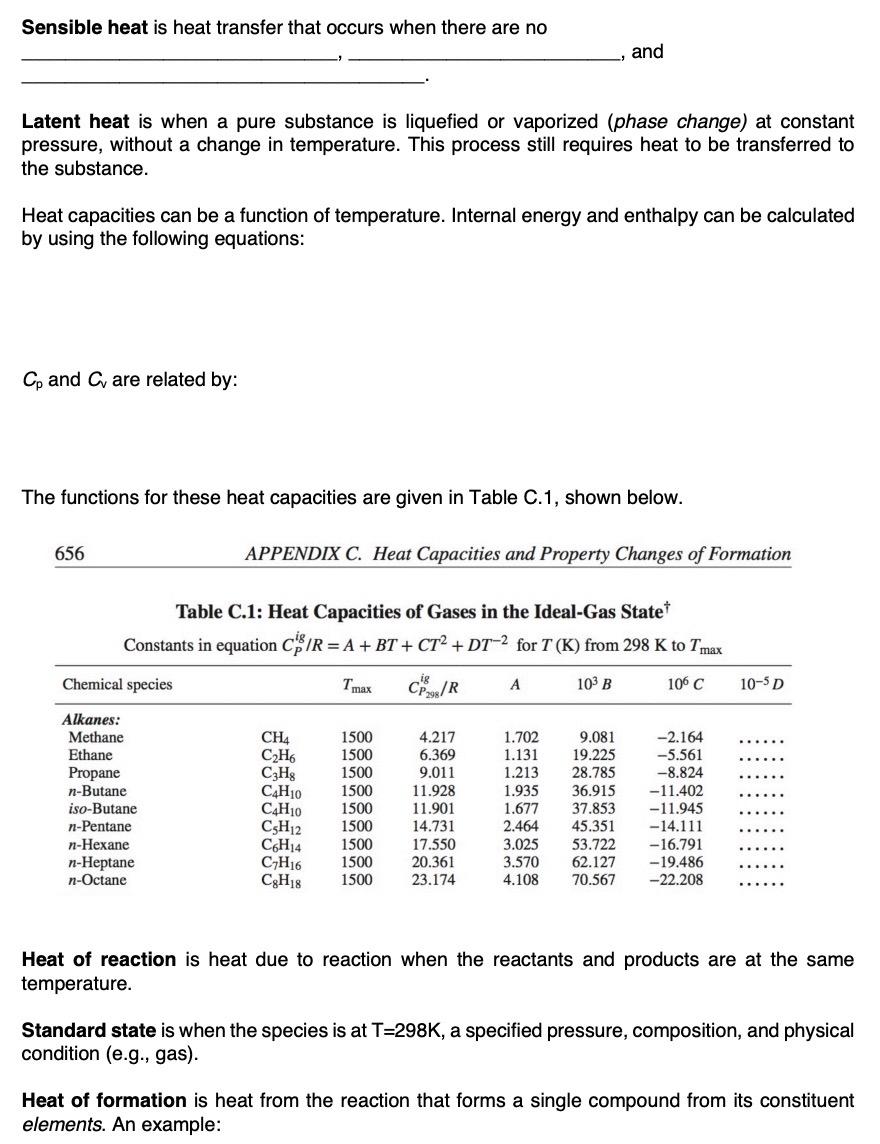
Sensible heat is heat transfer that occurs when there are no and Latent heat is when a pure substance is liquefied or vaporized (phase change) at constant pressure, without a change in temperature. This process still requires heat to be transferred to the substance. Heat capacities can be a function of temperature. Internal energy and enthalpy can be calculated by using the following equations: Cp and C are related by: The functions for these heat capacities are given in Table C.1, shown below. 656 APPENDIX C. Heat Capacities and Property Changes of Formation Table C.1: Heat Capacities of Gases in the Ideal-Gas State* Constants in equation C/R = A + BT + CT + DT-2 for T (K) from 298 K to Tmax Chemical species Tmax ig CP298/R A 103 B 106 C 10-5 D Alkanes: Methane CH4 1500 4.217 1.702 9.081 -2.164 Ethane C2H6 1500 6.369 1.131 19.225 -5.561 Propane C3H8 1500 9.011 1.213 28.785 -8.824 n-Butane C4H10 1500 11.928 1.935 36.915 -11.402 iso-Butane C4H10 1500 11.901 1.677 37.853 -11.945 n-Pentane C5H12 1500 14.731 2.464 45.351 -14.111 n-Hexane C6H14 1500 17.550 3.025 53.722 -16.791 n-Heptane C7H16 1500 20.361 3.570 62.127 -19.486 n-Octane C8H18 1500 23.174 4.108 70.567 -22.208 Heat of reaction is heat due to reaction when the reactants and products are at the same temperature. Standard state is when the species is at T-298K, a specified pressure, composition, and physical condition (e.g.. gas). Heat of formation is heat from the reaction that forms a single compound from its constituent elements. An example:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


