Answered step by step
Verified Expert Solution
Question
1 Approved Answer
show all work please Given: pH=7.1TotalAlkalinity=203mg/LasCaCO3TotalHardness=297mg/LasCaCO3CalciumHardness=235mg/LasCaCO3 The following guidelines for adding lime soda softening process chemicals are adanted from Nazaroff Note that all values in
show all work please 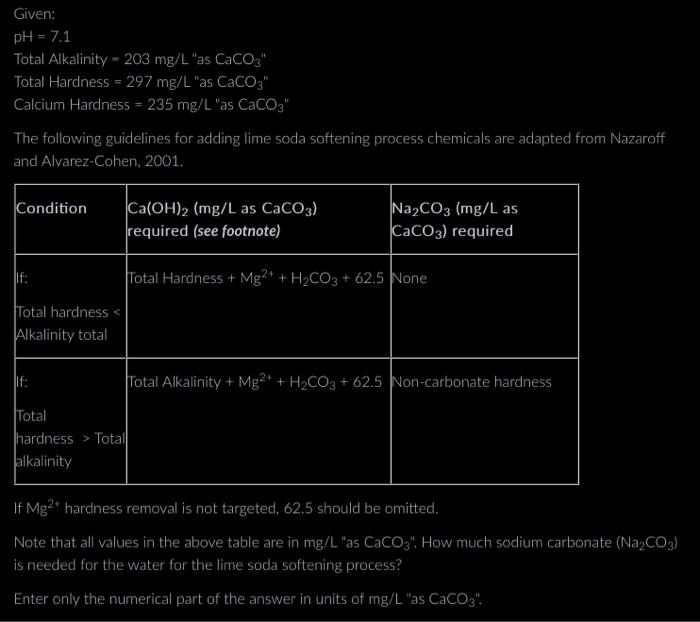
Given: pH=7.1TotalAlkalinity=203mg/L"asCaCO3TotalHardness=297mg/L"asCaCO3"CalciumHardness=235mg/L"asCaCO3" The following guidelines for adding lime soda softening process chemicals are adanted from Nazaroff Note that all values in the above table are in mg/L "as CaCO3 ". How much sodium carbonate (Na2CO3) is needed for the water for the lime soda softening process? Enter only the numerical part of the answer in units of mg/L "as CaCO3 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


