Answered step by step
Verified Expert Solution
Question
1 Approved Answer
show work please Problem 1 (20 points) Standard Heat of Reaction of the Oxidation of Sucrose Calculate the standard Heat of reaction for the oxidation
show work please 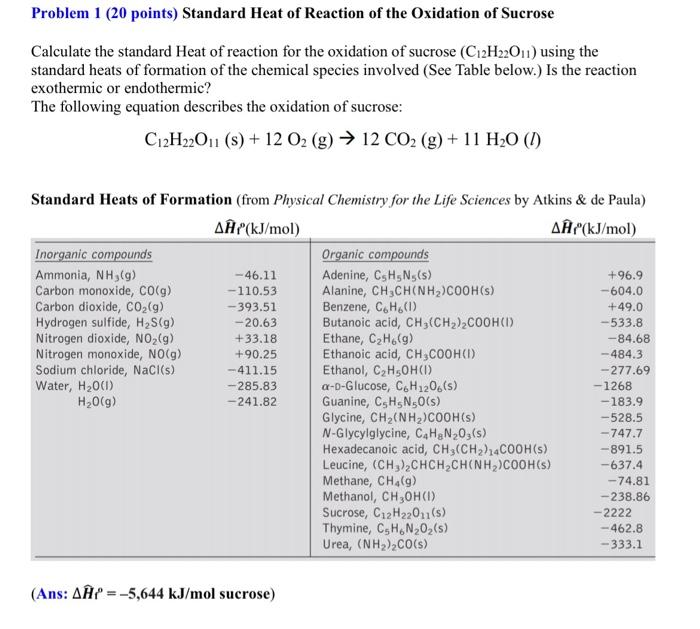
Problem 1 (20 points) Standard Heat of Reaction of the Oxidation of Sucrose Calculate the standard Heat of reaction for the oxidation of sucrose (C12H22011) using the standard heats of formation of the chemical species involved (See Table below.) Is the reaction exothermic or endothermic? The following equation describes the oxidation of sucrose: C12H22011 (8) + 12 O2 (g) 12 CO2 (g)+11 H20 (1) Standard Heats of Formation (from Physical Chemistry for the Life Sciences by Atkins & de Paula) Ah (kJ/mol) AA(kJ/mol) Inorganic compounds Organic compounds Ammonia, NH3(g) -46.11 Adenine, CsHsN5(s) +96.9 Carbon monoxide, CO(g) -110.53 Alanine, CHCH(NH)COOH(s) -604.0 Carbon dioxide, CO2(g) -393.51 Benzene, CH (1) +49.0 Hydrogen sulfide, H2S(g) --20.63 Butanoic acid, CH,(CH2)2COOH(D) -533.8 Nitrogen dioxide, NO2(g) +33.18 Ethane, C2H (9) -84.68 Nitrogen monoxide, NO(g) +90.25 Ethanoic acid, CH3COOH) -484.3 Sodium chloride, NaCl(s) -411.15 Ethanol, C2H5OH(1) -277.69 Water, H2O(1) -285.83 ar-D-Glucose, C6H120.(s) -1268 H2O(g) -241.82 Guanine, C.HSN 0(s) -183.9 Glycine, CH (NH2)COOH(s) -528.5 N-Glycylglycine, C4H9N203 (s) -747.7 Hexadecanoic acid, CH3(CH2) 14COOH(s) -891.5 Leucine, (CH3),CHCH2CH(NH)COOH(s) -637.4 Methane, CH (9) -74.81 Methanol, CH3OH (1) -238.86 Sucrose, C12H22011(s) -2222 Thymine, C.H.N202(s) -462.8 Urea, (NH2),CO(s) -333.1 (Ans: AAP = -5,644 kJ/mol sucrose) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


