Answered step by step
Verified Expert Solution
Question
1 Approved Answer
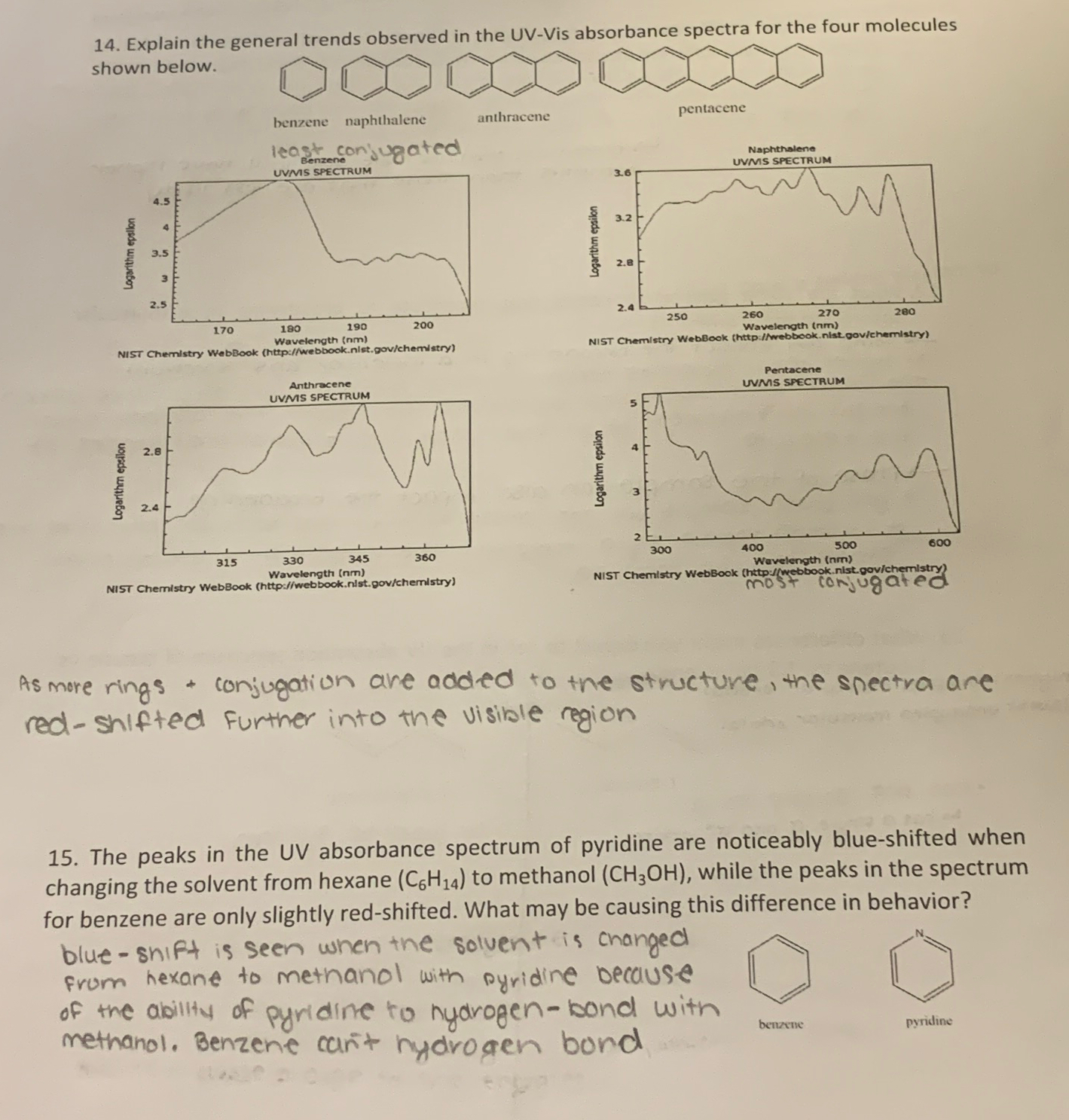
shown below. least conjuated As more rings + conjugation are added to the structure, the snectra are red - Shlfted further into the visible region
shown below.
least conjuated
As more rings conjugation are added to the structure, the snectra are redShlfted further into the visible region
The peaks in the UV absorbance spectrum of pyridine are noticeably blueshifted when changing the solvent from hexane to methanol while the peaks in the spectrum for benzene are only slightly redshifted. What may be causing this difference in behavior?
blueshift is seen when the solvent is changed from hexane to methanol with pyridine because of the ability of pyridine to nydrogenbond wits methanol. Benzene can't hydrogen bond
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


