Answered step by step
Verified Expert Solution
Question
1 Approved Answer
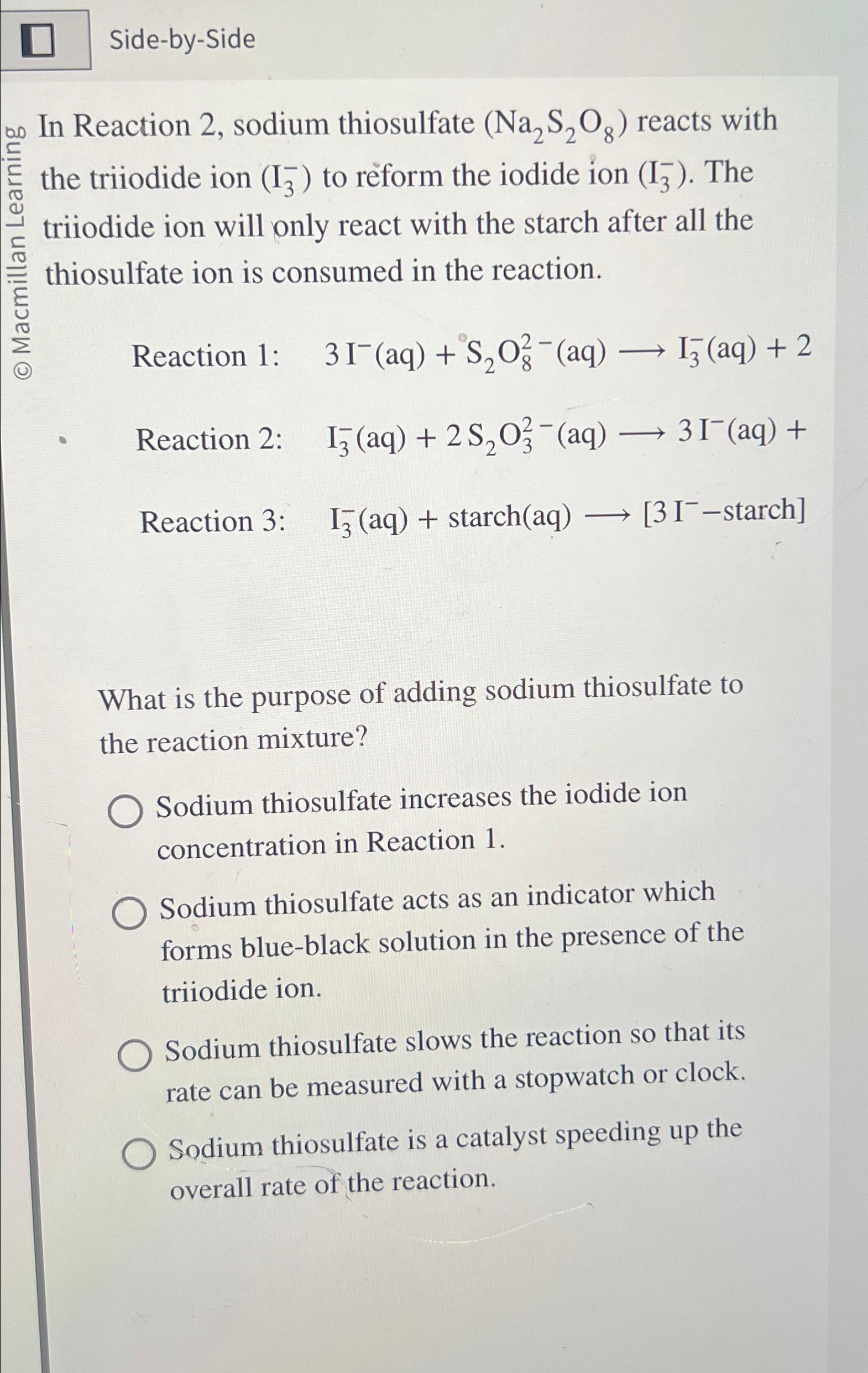
Side - by - Side In Reaction 2 , sodium thiosulfate ( N a 2 S 2 O 8 ) reacts with the triiodide ion
SidebySide
In Reaction sodium thiosulfate reacts with the triiodide ion to reform the iodide ion The triiodide ion will only react with the starch after all the thiosulfate ion is consumed in the reaction.
Reaction :
Reaction :
Reaction : starchstarch
What is the purpose of adding sodium thiosulfate to the reaction mixture?
Sodium thiosulfate increases the iodide ion concentration in Reaction
Sodium thiosulfate acts as an indicator which forms blueblack solution in the presence of the triiodide ion.
Sodium thiosulfate slows the reaction so that its rate can be measured with a stopwatch or clock.
Sodium thiosulfate is a catalyst speeding up the overall rate of the reaction.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


