Answered step by step
Verified Expert Solution
Question
1 Approved Answer
simultaneous determination of Caffeine and Aspirin in an analgesic by ultraviolet spectrophotometry. take the average of the two trials for your final conclusion. data from
simultaneous determination of Caffeine and Aspirin in an analgesic by ultraviolet spectrophotometry. 





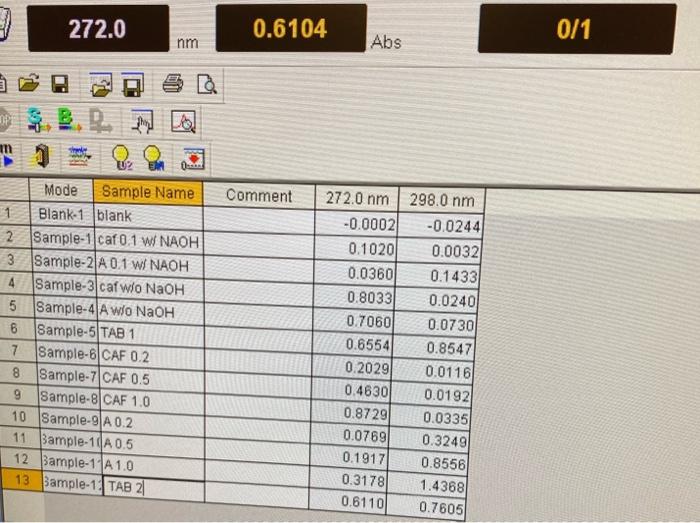
Purpose The simultaneous determination of caffeine and aspirin in a proprietary analgesic is performed by measuring absorbance values at two wavelengths and solving two equations in two unknowns. The milligrams of each per tablet are determined. Theory Aspirin and caffeine are common components of proprietary analgesics. Aspirin is an analgesic, antipyretic, and anti-inflammatory agent. Caffeine is sometime used as an adjuvant - to increase circulation and distribution of the primary active ingredient. Such products are subjected to strict quality control (QC) procedures to various instrumental within specified limits and safety. The procedures in intricular importance. components, where the components do Method. For a dilute mixture of two absorbing components, where the components do bech of not chemically interact in solution and where the absorbance spectra of eachentis display clearly defined and separate maxima, quantitative analysis of be analyzed by making based on Beer's law is feasible. A two-component mixture may be and 2, one for each absorbance measurements at two characteristic wavelengths ( is an where A1=a1,1bC1+a2,1bC2A2=a1,2bC1+a2,2bC2 A1= the total absorbance of the mixture at 1 A2= the total absorbance of the mixture at 2 a1,1= the absorptivity of component 1 at 1 a2,1= the absorptivity of component 2 at 1 a1,2= the absorptivity of component 1 at 2 a2,2= the absorptivity of component 2 at 2 C1= concentration of component 1 C2= concentration of component 2 b= the path length =1.0cm for the spectrophotometer and cuvettes used in this lab and is constant. It thus may be eliminated from consideration in the data analysis. Four calibration graphs are determined to yield the four absorptivity values: one for each compound (aspirin and caffeine) at each wavelength. Instrument (Granger, Sec. 6.4, p. 184-186). The spectrometer used in this experiment is a double-beam UV-vis spectrometer. The light sources (there are two-one for the visible wavelengths and one for the UV wavelengths) are split into two beams. One beam passes through a reference path and one through a sample path. The beams are recombined at different times (double beam in time) and sent to a transducer and operational amplifier (op-amp) circuit that detects the difference between the two signals. See Figure 6-20 in the text for a diagram of a double beam in space spectrophotometer. More precise measurements may be made with double beam spectrometers than with single beam instruments, primarily because any variation in source lamp intensity with time is automatically subtracted and does not affect the analytical signal. Solution preparations A. Prepare standard solutions of acetylsalicylic acid ( 375mg) and caffeine ( 95mg) by dissolving each in separate 50mL solutions of methanol, adding 10 drops of 4M NaOH, and warming gently for 15min, in a small, covered beaker (be careful, methanol boils at 65C and is flammable). Make sure the masses are recorded accurately to 4 sig figs with an analytical balance to know the standard concentrations accurately. Cool, quantitatively transfer, and dilute each to 250mL with methanol in a volumetric flask. Dilute 0.1,0.2,0.5, and 1.0mL aliquots of each standard into separate 25-mL volumetric flasks with methanol. You should have 4 well-defined standards for both acetylsalicylic acid and caffeine ( 8 solutions total). Pure methanol will serve as a blank. B. Prepare separate solutions of caffeine and aspirin (not necessary to be very accurate) in methanol only (no NaOH ) for a separate side experiment. Use 75 mg aspirin and 19 mg caffeine in 50mL methanol, then dilute 2mL of these solutions to 50mL. These will only be measured to see what effect NaOH has on the spectra of aspirin and caffeine. C. Weigh a proprietary analgesic tablet containing both acetylsalicylic acid and caffeine (e.g. Anacin). Crush and grind the tablet with a clean mortar and pestle. Transfer as quantitatively as possible to weighing paper and determine the mass of powder that will be analyzed. Transfer this sample to a 100mL beaker. Repeat with a second tablet separately into a second 100mL beaker to provide two samples. Dissolve each in 50 mL of methanol, adding 10 drops of 4MNaOH and gently warming for 15 min stirring with a magnetic stir bar. Binding agents used in the manufacturing process will remain undissolved. After cooling, quantitatively transfer to a 100-mL volumetric flask. Dilute to the mark with methanol and mix thoroughly. Let undissolved particles fully settle. You may leave this in your drawer until next period to let them fully settle, or you may centrifuge a portion to analyze. Dilute 0.5mL of the supernatent accurately to 50mL with methanol in a volumetric flask. If the undissolved particles are colloidal (extremely small, 





take the average of the two trials for your final conclusion.
data from experiment:
0.3783 g Aspirin
0.0940 g Caffeine
tablet 1 mass: 0.5347g
mass of powder 1: 0.5130g
tablet 2 mass: 0.5287g
mass of powder 2: 0.4178g

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


