Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Sodium hypochlorite is formed according to the reaction 2NaOH + Cl2NaOCI + NaCl + HO in a continuous reactor by bubbling Cl, through a
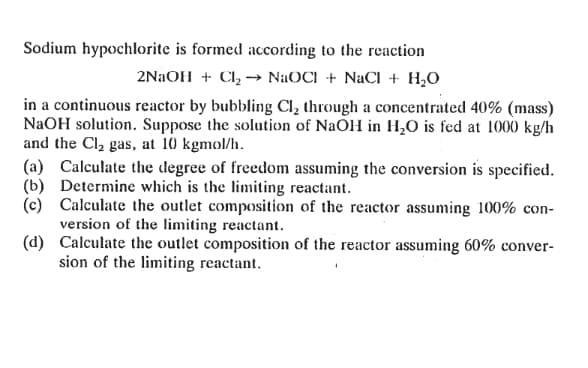
Sodium hypochlorite is formed according to the reaction 2NaOH + Cl2NaOCI + NaCl + HO in a continuous reactor by bubbling Cl, through a concentrated 40% (mass) NaOH solution. Suppose the solution of NaOH in HO is fed at 1000 kg/h and the Cl gas, at 10 kgmol/h. (a) Calculate the degree of freedom assuming the conversion is specified. (b) Determine which is the limiting reactant. (c) Calculate the outlet composition of the reactor assuming 100% con- version of the limiting reactant. (d) Calculate the outlet composition of the reactor assuming 60% conver- sion of the limiting reactant.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


