Answered step by step
Verified Expert Solution
Question
1 Approved Answer
solution is formed when the solute uniformly disperses oughout (or dissolves in) the solvent. The process can be scribed though three steps: 1. separation
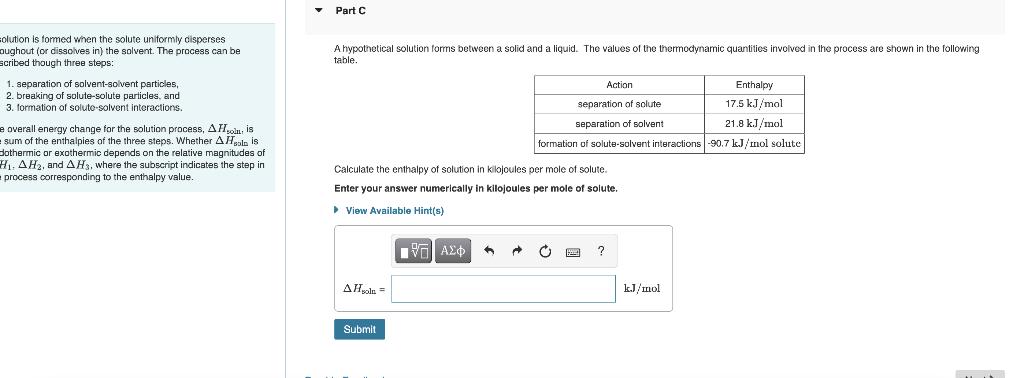
solution is formed when the solute uniformly disperses oughout (or dissolves in) the solvent. The process can be scribed though three steps: 1. separation of solvent-solvent particles, 2. breaking of solute-solute particles, and 3. formation of soluto-solvent interactions. e overall energy change for the solution process, A His sum of the enthalpies of the three steps. Whether A Hol is dothermic or exothermic depends on the relative magnitudes of H. AH2, and AH3, where the subscript indicates the step in process corresponding to the enthalpy value. Part C A hypothetical solution forms between a solid and a liquid. The values of the thermodynamic quantities involved in the process are shown in the following table. Action Enthalpy 17.5 kJ/mol separation. solute separation of solvent 21.8 kJ/mol formation of solute-solvent interactions -90.7 kJ/mol solute Calculate the enthalpy of solution in kilojoules per mole of solute. Enter your answer numerically in kilojoules per mole View Available Hint(s) solute. A C ? AHsola = Submit kJ/mol
Step by Step Solution
★★★★★
3.50 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Solution By definition bond breaking required ener...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


