Answered step by step
Verified Expert Solution
Question
1 Approved Answer
solve quickly for thumbs up answer is (a) For a reference temperature of 25C, calculate the mean heat capacity Cpm for CO at 600C. For
solve quickly for thumbs up 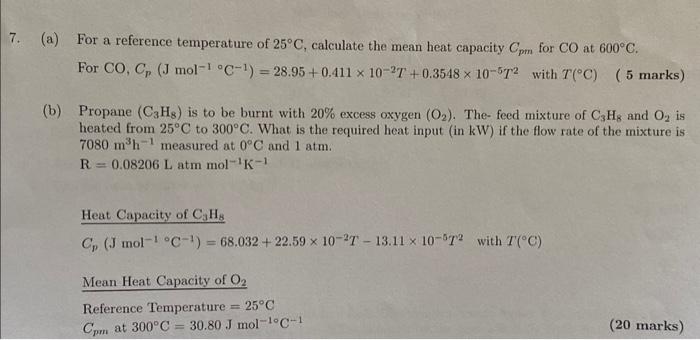
(a) For a reference temperature of 25C, calculate the mean heat capacity Cpm for CO at 600C. For CO,Cp(Jmol1C1)=28.95+0.411102T+0.3548105T2 with T(C) ( 5 marks) (b) Propane (C3H8) is to be burnt with 20% excess oxygen (O2). The-feed mixture of C3H8 and O2 is heated from 25C to 300C. What is the required heat input (in kW ) if the flow rate of the mixture is 7080m3h1 measured at 0C and 1atm. R=0.08206Latmmol1K1 Heat Capacity of C3H8 Cp(Jmol1C1)=68.032+22.59102T13.11105T2 with T(C) Mean Heat Capacity of O2 Reference Temperature =25C Cpm at 300C=30.80Jmol1C1 (20 marks) (a) Cpm=30.68Jmol1C (b) Q=983kW 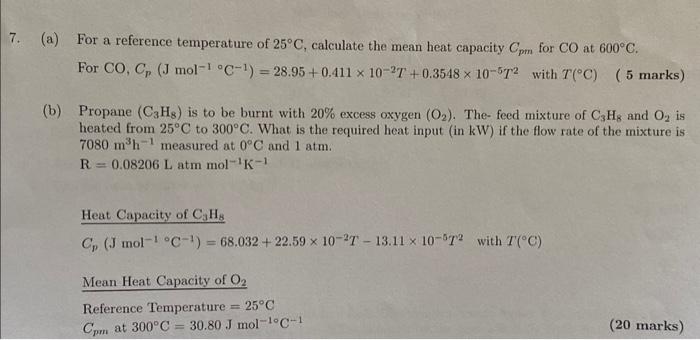
answer is 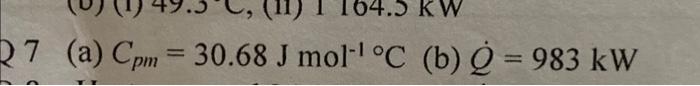
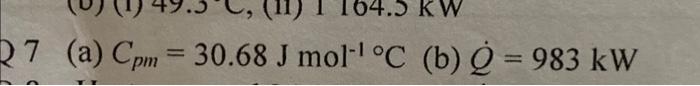
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


