Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Solve table and Use titration data from Table 1 to calculate the effective molarity of the iodate titrant (show calculations from both trials). o Use
Solve table and Use titration data from Table 1 to calculate the effective molarity of the iodate titrant
(show calculations from both trials).
o Use titration data from Table 2 to determine the mass of ascorbic acid in a packet of Emergen-C Super Orange^tm (show calculations from both trials)
o Determine the percent difference (aka % error) between the quantity of vitamin C determined experimentally and the quantity indicated on the Emergen-C Super
Orange^tm packaging
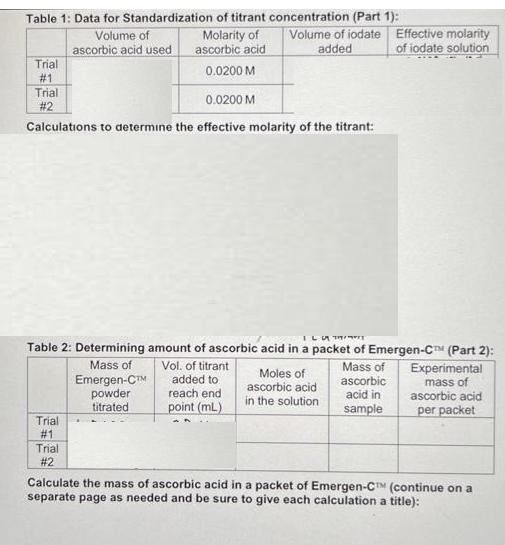
Table 1: Data for Standardization of titrant concentration (Part 1): Molarity of ascorbic acid 0.0200 M 0.0200 M Calculations to determine the effective molarity of the titrant: Trial #1 Trial #2 Trial #1 Volume of ascorbic acid used Table 2: Determining amount of ascorbic acid in a packet of Emergen-CTM (Part 2): Mass of ascorbic acid in sample Trial #2 Mass of Emergen-C powder titrated Volume of iodate Effective molarity added of iodate solution Vol. of titrant added to reach end point (mL) Moles of ascorbic acid in the solution Experimental mass of ascorbic acid per packet Calculate the mass of ascorbic acid in a packet of Emergen-CTM (continue on a separate page as needed and be sure to give each calculation a title):
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Trial 1 RTO Molarity of ascorbic acid x Volume of ascorbic acid used 00200 M x 00200 L 0000400 mol E...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


