Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Solve this problem step by step answer please only hand written accepted a) A reversible heat engine operates between 700 K and 300 K and
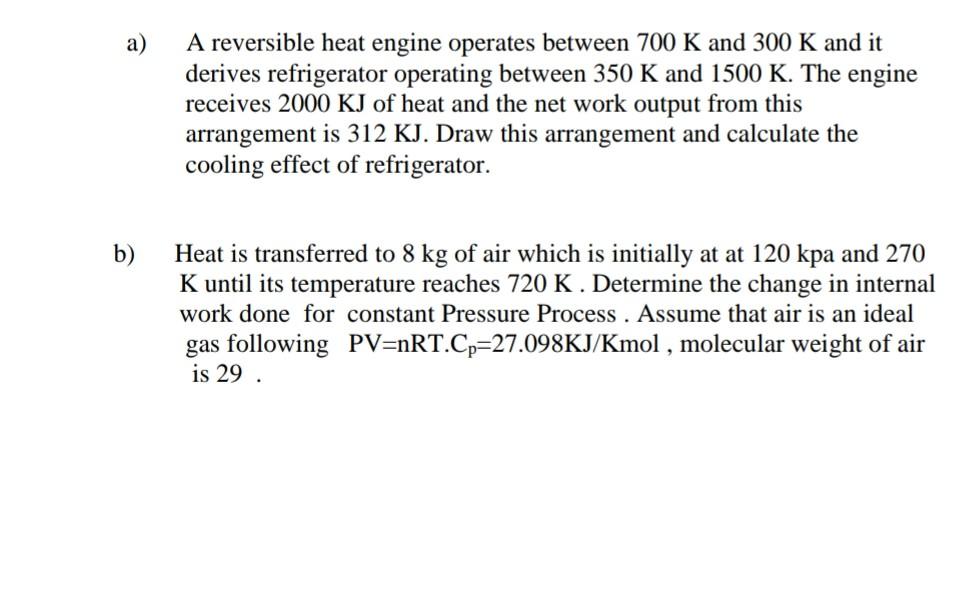
Solve this problem step by step answer please only hand written accepted
a) A reversible heat engine operates between 700 K and 300 K and it derives refrigerator operating between 350 K and 1500 K. The engine receives 2000 KJ of heat and the net work output from this arrangement is 312 KJ. Draw this arrangement and calculate the cooling effect of refrigerator. b) Heat is transferred to 8 kg of air which is initially at at 120 kpa and 270 K until its temperature reaches 720 K. Determine the change in internal work done for constant Pressure Process . Assume that air is an ideal gas following PV=nRT.Cp=27.098KJ/Kmol , molecular weight of air is 29Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


