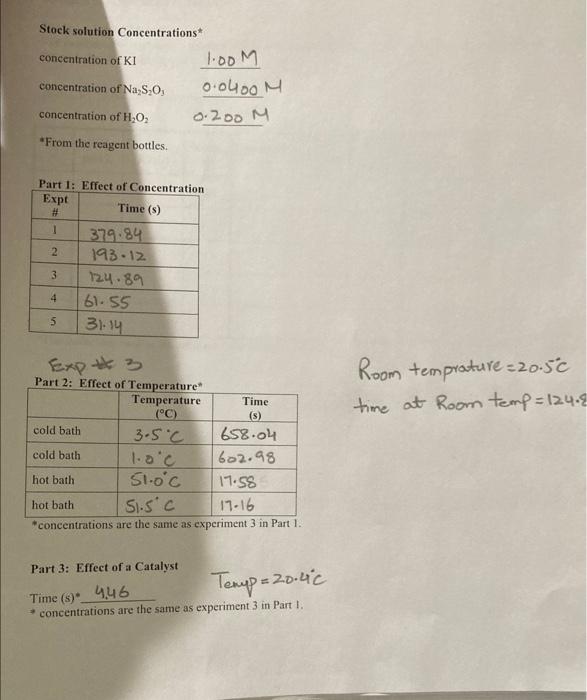
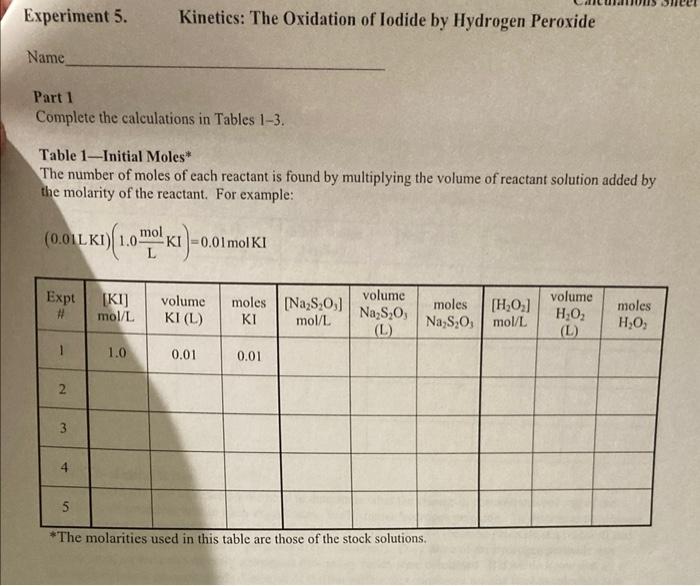
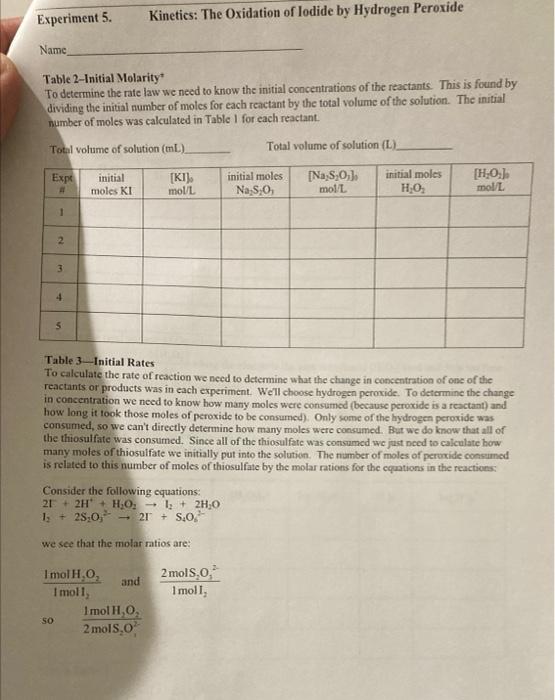
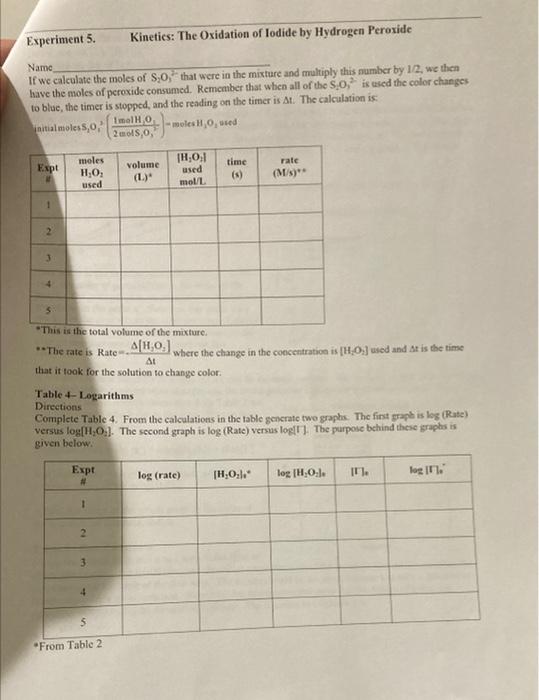
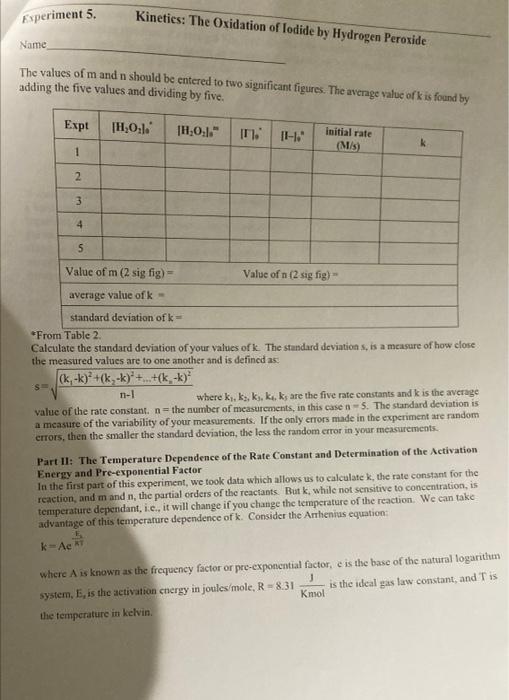
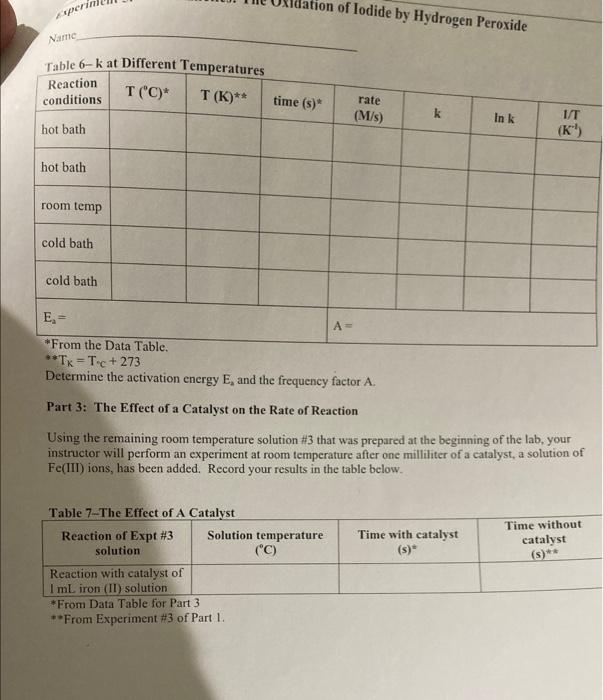
Stock solution Concentrations concentration of KI 1.00M concentration of Na S,O, 0.000M concentration of H,O, 0.200 M From the reagent bottles. Part 1: Effect of Concentration Expt Time (s) # 1 379.84 2 193.12 3 124.89 4 61-55 5 3-14 Room temprature=20.5c time at Room temp=124.8 Expt 3 Part 2: Effect of Temperature Temperature Time (C) (5) cold bath 3.5C 658.04 cold bath 1.oc 602.98 hot bath 51.0c 17.58 hot bath 51.5 c 17-16 *concentrations are the same as experiment 3 in Part 1. Part 3: Effect of a Catalyst Temp = 20.60 Time (s) 446 concentrations are the same as experiment 3 in Part I. Experiment 5. Kinetics: The Oxidation of lodide by Hydrogen peroxide Name Part 1 Complete the calculations in Tables 1-3. Table 1-Initial Moles* The number of moles of each reactant is found by multiplying the volume of reactant solution added by the molarity of the reactant. For example: (0.01LKI)(1.0 mo! meI) KI -0.01 mol KI Expt [KI] mol/L volume KI(L) moles (Na2S 0,1 KI mol/L volume Na S,O, moles [H0] Na S.O,mol/L volume HO (L.) moles HO (L 1.0 0.01 0.01 2 3 4 5 * The molarities used in this table are those of the stock solutions Experiment 5. Kinetics: The Oxidation of lodide by Hydrogen Peroxide Name Table 2-Initial Molarity To determine the rate law we need to know the initial concentrations of the reactants. This is found by dividing the initial number of moles for each reactant by the total volume of the solution. The initial number of moles was calculated in Table 1 for each reactant. Total volume of solution (m) Total volume of solution (L). Expe initial molcs KI [KI) mol/L initial moles Na S:0 [Na S,O] mol/L initial moles HO [HO] mol/L 1 2 3 4 5 Table 3 Initial Rates To calculate the rate of reaction we need to determine what the change in concentration of one of the reactants or products was in each experiment. We'll choose hydrogen peroxide. To determine the change in concentration we need to know how many moles were consumed (because peroxide is a reactant) and how long it took those moles of peroxide to be consumed). Only some of the hydrogen peroxide was consumed, so we can't directly determine how many moles were consumed. But we do know that all of the thiosulfate was consumed. Since all of the thiosulfate was consumed we just need to calculate how many moles of thiosulfate we initially put into the solution. The number of moles of perurtide consumed is related to this number of moles of thiosulfate by the molar rations for the equations in the reactions Consider the following equations: 21 + 2H+H.O. - 1 + 2H.0 1. + 28,0, - 2 + 5.02 we see that the molar ratios are: 2mols,o, I moll, Imol H,O, and Imoll, Imol H,O 2 mols. SO Experiment 5. Kinetics: The Oxidation of lodide by Hydrogen peroxide Name If we calculate the moles of S.O, that were in the mixture and multiply this number by 12, we then have the moles of peroxide consumed. Remember that when all of the S.O," is used the color changes to blue, the timer is stopped, and the reading on the timer is At. The calculation is: initial moles 5,02 Imelho eles H Owned 2mols,o, Expt moles H,O, used volume (L.)" 1H,0:1 used mol/L time (5) rate (M) 1 2 3 4 4 This is the total volume of the mixture **The rate is Rates A[1.0.1 where the change in the concentration is [H0] used and it is the time that it took for the solution to change color Table - Logarithms Directions Complete Table 4. From the calculations in the table generate two graphs. The first graph is log (Rate) versus log[10]. The second graph is log (Rate) versus log!!)The purpose behind these graphs is given below. Expt log (rate) [H2O: log (H.O.l. IT. login. 1 2 3 5 From Table 2 Kinetics: The Oxidation of lodide by Hydrogen peroxide Experiment 5. Name The values of m and n should be entered to two significant figures. The average value of k is found by adding the five values and dividing by five. Expt TH,0:1. 1H,0:1." 1. initial rate (M/s) K 1 2 3 4 5 Value of m (2 sig fig) Value of n (2 sig fig) average value of k- standard deviation of k= "From Table 2 Calculate the standard deviation of your values of k The standard deviations, is a measure of how close the measured values are to one another and is defined as: (k, -k)* +(k-k) +...+(k-k)? $ where k.ks, ks, ka,ks are the five rate constants and k is the average value of the rate constant, n= the number of measurements, in this case n-5. The standard deviation is a measure of the variability of your measurements. If the only crrors made in the experiment are random errors, then the smaller the standard deviation, the less the random error in your measurements. Part II: The Temperature Dependence of the Rate Constant and Determination of the Activation Energy and Pre-exponential Factor In the first part of this experiment, we took data which allows us to calculatek, the rate constant for the reaction, and m and n, the partial orders of the reactants. But k, while not sensitive to concentration, is temperature dependant, i.e., it will change if you change the temperature of the reaction. We can take advantage of this temperature dependence of t. Consider the Anthenius equation: kAc where A is known as the frequency factor or pre-exponential factor, e is the base of the natural logarithm system, E, is the activation energy in joules/mole, R = 8.31 is the ideal gas law constant, and is Knol the temperature in kelvin perin tion of lodide by Hydrogen Peroxide Name Table 6-k at Different Temperatures Reaction conditions T("C)* T(K)** time (5) rate (M/s) k In k hot bath (K) hot bath room temp cold bath cold bath E- A= *From the Data Table. **TK = T. + 273 Determine the activation energy E, and the frequency factor A. Part 3: The Effect of a Catalyst on the Rate of Reaction Using the remaining room temperature solution #3 that was prepared at the beginning of the lab. your instructor will perform an experiment at room temperature after one milliliter of a catalyst, a solution of Fe(III) ions, has been added. Record your results in the table below. Time with catalyst (s) Time without catalyst (s)** Table 7-The Effect of A Catalyst Reaction of Expt #3 Solution temperature solution ("C) Reaction with catalyst of I ml iron (II) solution *From Data Table for Part 3 **From Experiment #3 of