Answered step by step
Verified Expert Solution
Question
1 Approved Answer
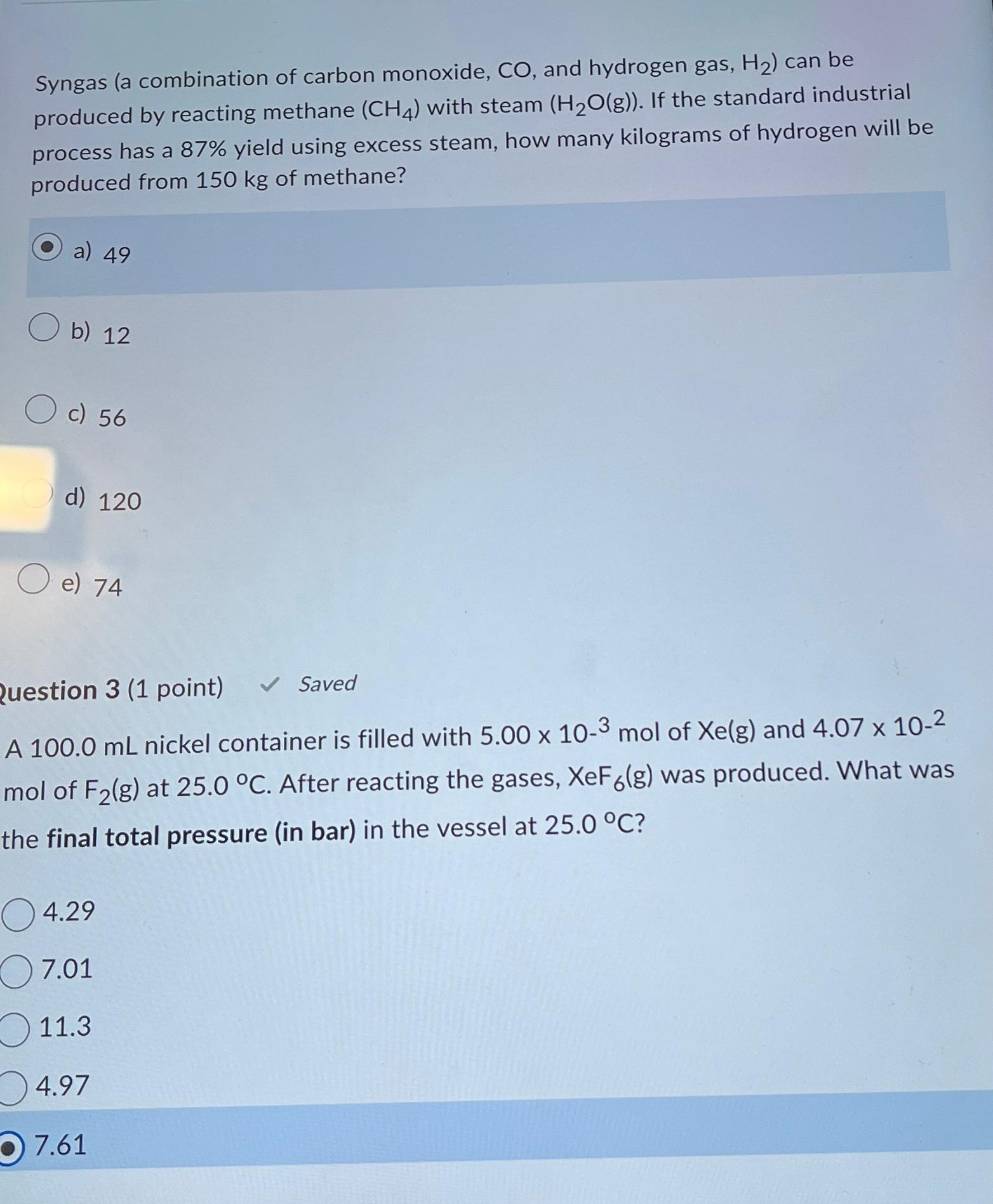
Syngas ( a combination of carbon monoxide, C O , and hydrogen gas, H 2 ) can be produced by reacting methane ( C H
Syngas a combination of carbon monoxide, and hydrogen gas, can be produced by reacting methane with steam If the standard industrial process has a yield using excess steam, how many kilograms of hydrogen will be produced from of methane?
a
b
c
d
e
Question point
Saved
A nickel container is filled with mol of and mol of at After reacting the gases, was produced. What was the final total pressure in bar in the vessel at
Solve both with full steps please.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


