Answered step by step
Verified Expert Solution
Question
1 Approved Answer
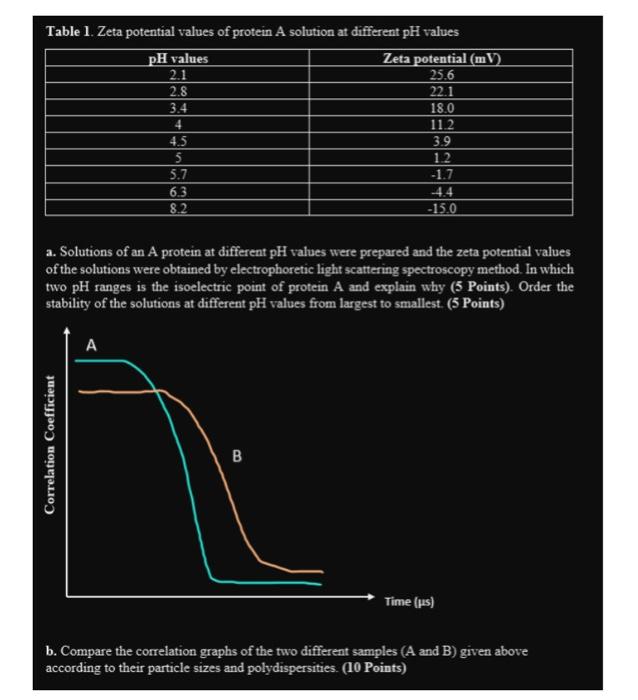
Table 1. Zeta potential values of protein A solution at different pH values pH values Zeta potential (mV) 25.6 2.1 2.8 22.1 3.4 18.0 4
Table 1. Zeta potential values of protein A solution at different pH values pH values Zeta potential (mV) 25.6 2.1 2.8 22.1 3.4 18.0 4 Correlation Coefficient 4.5 5 5.7 6.3 8.2 A a. Solutions of an A protein at different pH values were prepared and the zeta potential values of the solutions were obtained by electrophoretic light scattering spectroscopy method. In which two pH ranges is the isoelectric point of protein A and explain why (5 Points). Order the stability of the solutions at different pH values from largest to smallest. (5 Points) 11.2 3.9 1.2 -1.7 B -4.4 -15.0 Time (us) b. Compare the correlation graphs of the two different samples (A and B) given above according to their particle sizes and polydispersities. (10 Points)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


