Tabulated below are initial rate data for the chemical reaction: 2Fe(CN)6 +212Fe(CN)6+1 Run 1 2 3 4 5 [Fe(CN) lo 0.01 0.01 0.02 0.02
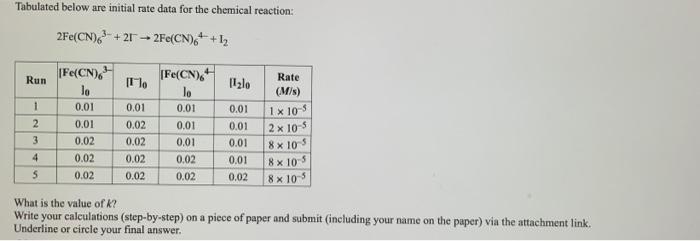
Tabulated below are initial rate data for the chemical reaction: 2Fe(CN)6 +212Fe(CN)6+1 Run 1 2 3 4 5 [Fe(CN) lo 0.01 0.01 0.02 0.02 0.02 [o 0.01 0.02 0.02 0.02 0.02 [Fe(CN) 0.01 0.01 0.01 0.02 0.02 [lo Rate (M/s) 0.01 0.01 0.01 0.01 0.02 8 x 10-5 1x 10-5 2x 10-5 8 x 10-5 8x10-5 What is the value of k? Write your calculations (step-by-step) on a piece of paper and submit (including your name on the paper) via the attachment link. Underline or circle your final answer.
Step by Step Solution
3.45 Rating (171 Votes )
There are 3 Steps involved in it
Step: 1
from run 1 and 2 only 1 doubles rate doubles So order of l is 1 from run 2 and 3 ...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


