Answered step by step
Verified Expert Solution
Question
1 Approved Answer
thank ypu for your help. The liquid-phase irreversible reaction AB+C is carried out in an ideal constant volume batch reactor. The concentrations of specie A
thank ypu for your help. 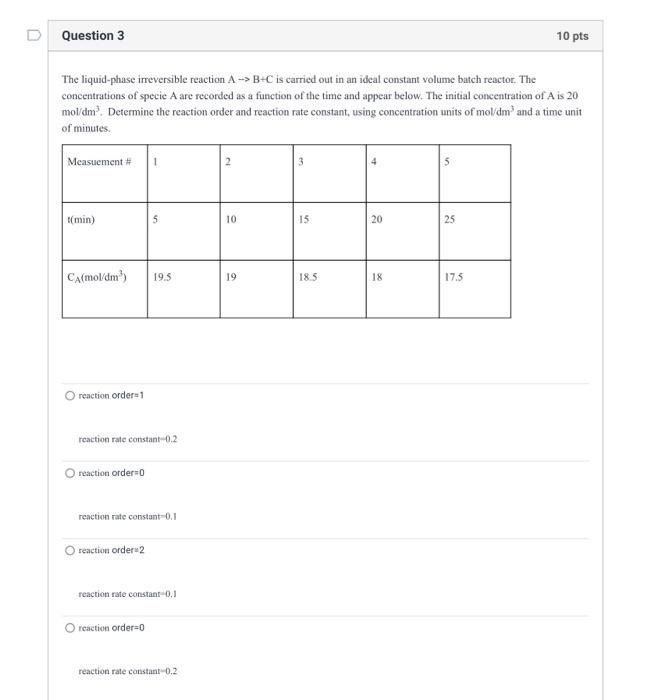
The liquid-phase irreversible reaction AB+C is carried out in an ideal constant volume batch reactor. The concentrations of specie A are recorded as a function of the time and appear below. The initial concentration of A is 20 mol /dm3. Determine the reaction order and reaction rate constant, using concentration units of mol/m 3 and a time unit of minutes. renction order 1 reaction rate constant -0.2 reaction order =0 reaction rate constant =0.1 reaction orderu 2 reaction rate constant -0.1 reaction order =0 reaction rate constant -0.2 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


