Answered step by step
Verified Expert Solution
Question
1 Approved Answer
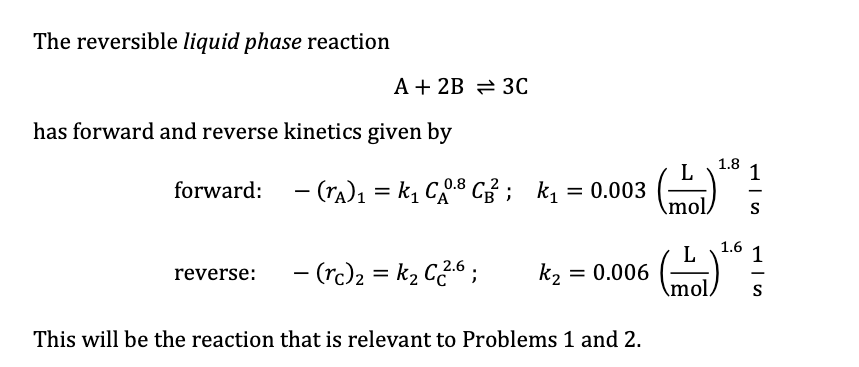
The above reaction is carried out in a batch reactor of constant liquid volume and temperature. Initially ( at t = 0 ) , the
The above reaction is carried out in a batch reactor of constant liquid volume and
temperature. Initially at the concentrations of the three components are
;;
a Calculate and by integrating the mole balance equations over time.
Plot all three concentrations on the same graph, with time on the horizontal axis
ranging from to
To produce reasonably smooth curves, your time intervals within the first seconds
should be quite small eg
b What are the concentrations and at time
c What is the value of at which is effectively
d Confirm your answer to part c using an approach that does not involve integration of
ODEs.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


