Question
The acid-catalyzed hydrolysis of sucrose occurs by the following overall reaction whose kinetic data are given below: C 12 H 2 O 11 (s) +H
The acid-catalyzed hydrolysis of sucrose occurs by the following overall reaction whose kinetic data are given below:
C 12 H 2 O 11 (s) +H 2 O (l) ⟶C 6 H 12 O 6 (aq) +C 6 H 12 O 6(aq)
sucrose ⟶ glucose fructose
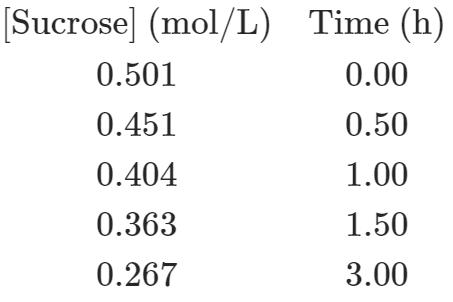
(a) Determine the rate constant and the half-life of the reaction.
(b) How long does it take to hydrolyze 75% of the sucrose?
(c) Other studies have shown that this reaction is actually second-order overall but appears to follow first-order kinetics. (Such a reaction is called a pseudo-first-order reaction.) Suggest a reason for this apparent first-order behavior.
[Sucrose] (mol/L) Time (h) 0.501 0.00 0.451 0.50 0.404 1.00 0.363 1.50 0.267 3.00
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
a from graph it is first order reaction lnsucrose Rtc we get l...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Materials Science and Engineering An Integrated Approach
Authors: David G. Rethwisch
4th Edition
1118214226, 1118061608, 9781118214220, 978-1118061602
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



