Question
The analysis of a coal indicates 60 wt% C, 35% H, 2% S, and the balance noncombustible ash. The coal is burned at a rate
The analysis of a coal indicates 60 wt% C, 35% H, 2% S, and the balance noncombustible ash. The coal is burned at a rate of 300,000 kg/h, and pure oxygen is fed with 5% in excess. All of the noncombustible ash and 20% of the carbon in the fuel leave the furnace as a molten slag; the remainder of the carbon leaves in the stack gas as CO and CO2; the hydrogen in the coal is oxidized to water, and the sulfur emerges as SO2. The selectivity of C02 to CO production is 5:1. (MW C=12, H=1, S=32.1, O=16). Calculate the flow rate in ft3/min of oxygen not used in this process at 35 psig, and 68F
.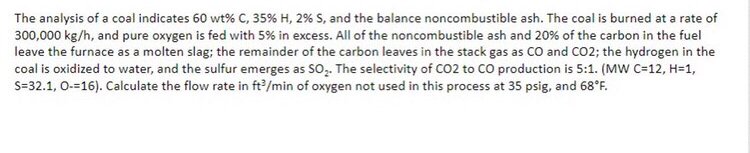
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


