Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The answer can be short. In addition, please answer all questions, and do not copy other people's answers to me , I will give you
The answer can be short. In addition, please answer all questions, and do not copy other people's answers to me, I will give you a good evaluation based on the answer.
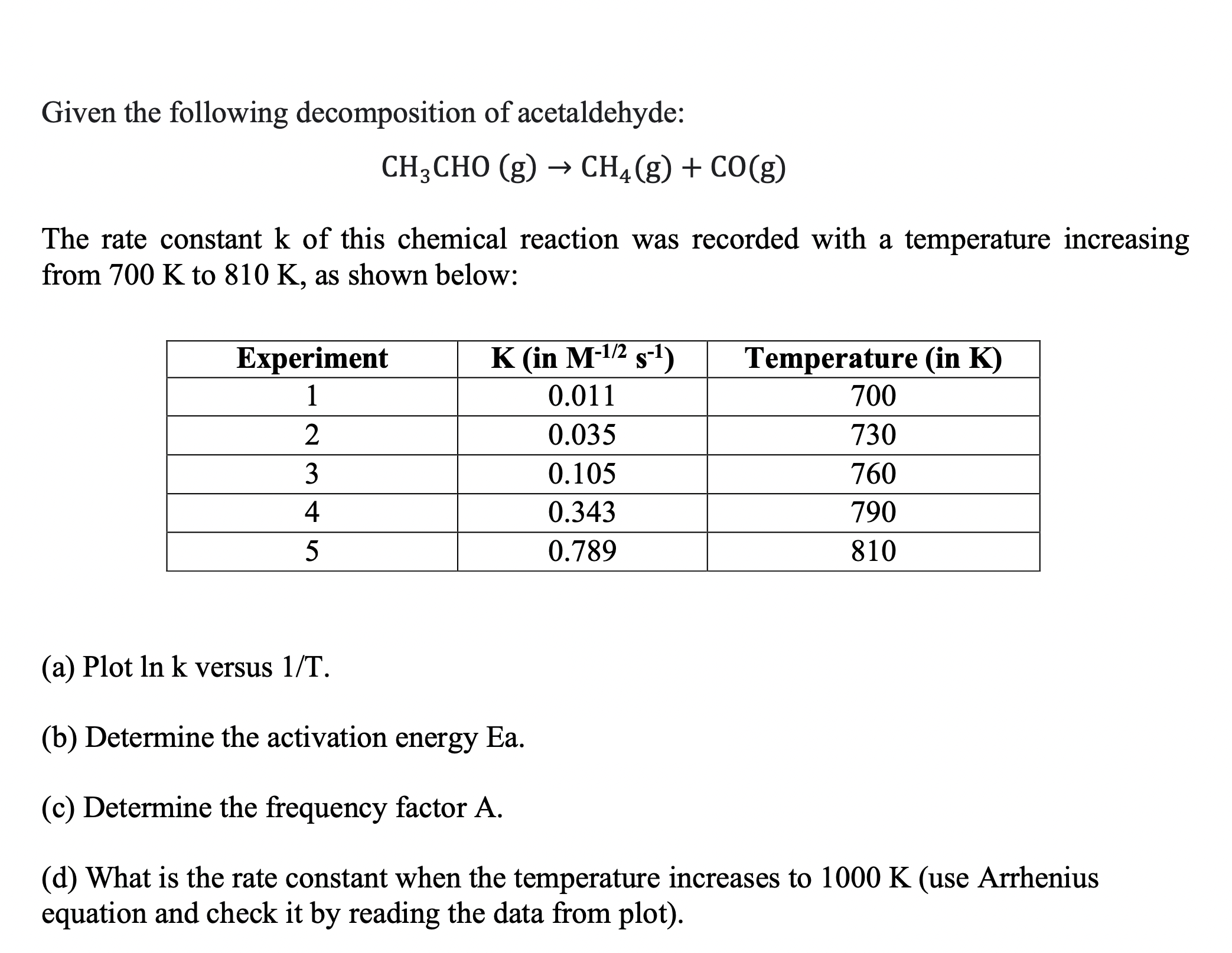 Given the following decomposition of acetaldehyde: CH3CHO(g)CH4(g)+CO(g) The rate constant k of this chemical reaction was recorded with a temperature increasing from 700K to 810K, as shown below: (a) Plot lnk versus 1/T. (b) Determine the activation energy Ea. (c) Determine the frequency factor A. (d) What is the rate constant when the temperature increases to 1000K (use Arrhenius equation and check it by reading the data from plot)
Given the following decomposition of acetaldehyde: CH3CHO(g)CH4(g)+CO(g) The rate constant k of this chemical reaction was recorded with a temperature increasing from 700K to 810K, as shown below: (a) Plot lnk versus 1/T. (b) Determine the activation energy Ea. (c) Determine the frequency factor A. (d) What is the rate constant when the temperature increases to 1000K (use Arrhenius equation and check it by reading the data from plot) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


