Answered step by step
Verified Expert Solution
Question
1 Approved Answer
the bottom has clear photos 3.37 How many total electrons reside in Mos of symmetry in the following cation? 3.39 of the wonderwerpen 3.41 (a)
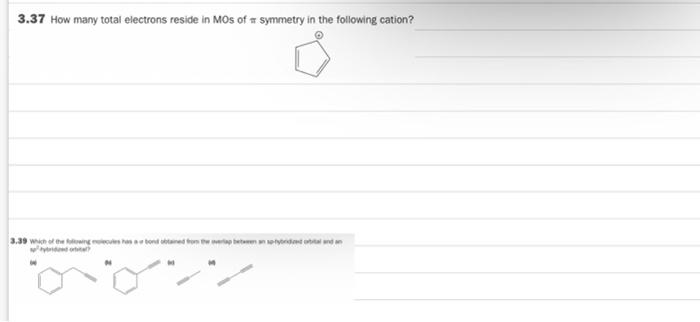
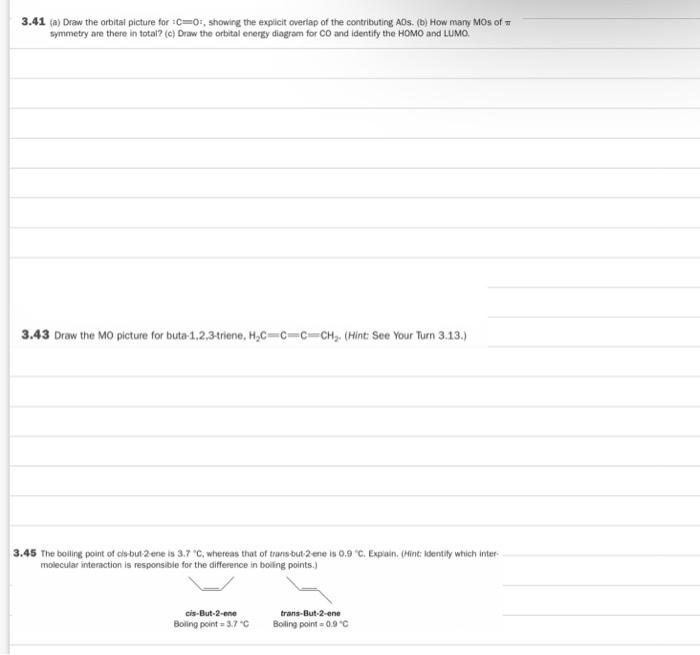

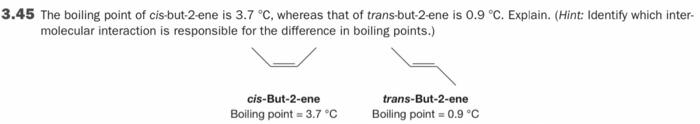

the bottom has clear photos
3.37 How many total electrons reside in Mos of symmetry in the following cation? 3.39 of the wonderwerpen 3.41 (a) Draw the orbital picture for C=0, showing the explicit overlap of the contributing AOS. (b) How many Mos of symmetry are there in total? (c) Draw the orbital energy diagram for CO and identify the HOMO and LUMO 3.43 Draw the MO picture for buta-1,2,3-triene, Hc-c-C-CH. (Hint: See Your Turn 3.13.) 3.45 The bolling point of cls but 2 ene is 3.7 C, whereas that of transdut2 ene is 0.9 "C. Expain. (Hint Identity which inter molecular interaction is responsible for the difference in boiling points.) cis-But-2-ene Boling point 3.70 trans-But-2-ene Boiling point 0.9C 3.37 How many total electrons reside in MOs of symmetry in the following cation? 3.45 The boiling point of cis-but-2-ene is 3.7 C, whereas that of trans-but-2-ene is 0.9 C. Explain. (Hint: Identify which inter- molecular interaction is responsible for the difference in boiling points.) cis-But-2-ene Boiling point - 3.7C trans-But-2-ene Boiling point = 0.9 C 3.41 (a) Draw the orbital picture for :C=0, showing the explicit overlap of the contributing AOs. (b) How many MOs of symmetry are there in total? (c) Draw the orbital energy diagram for CO and identify the HOMO and LUMO Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


