Question
200 m of a gaseous mix comprising 80% acetylene - 20% inerts stream (molar basis) measured at 550C and 20 atm are to be
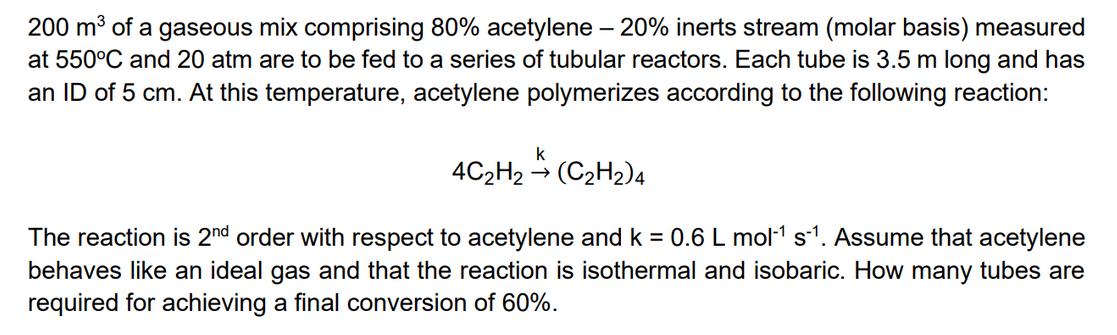
200 m of a gaseous mix comprising 80% acetylene - 20% inerts stream (molar basis) measured at 550C and 20 atm are to be fed to a series of tubular reactors. Each tube is 3.5 m long and has an ID of 5 cm. At this temperature, acetylene polymerizes according to the following reaction: k -> 4C2H2 (C2H2)4 The reaction is 2nd order with respect to acetylene and k = 0.6 L mol-1 s-1. Assume that acetylene behaves like an ideal gas and that the reaction is isothermal and isobaric. How many tubes are required for achieving a final conversion of 60%.
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
To calculate the number of tubes required to achieve a final conversion of 60 for the polymerization ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Marketing Strategy
Authors: O. C. Ferrell, Michael Hartline
6th edition
1285073045, 978-1285607139, 1285607139, 978-1285677316, 978-1285073040
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



