Answered step by step
Verified Expert Solution
Question
1 Approved Answer
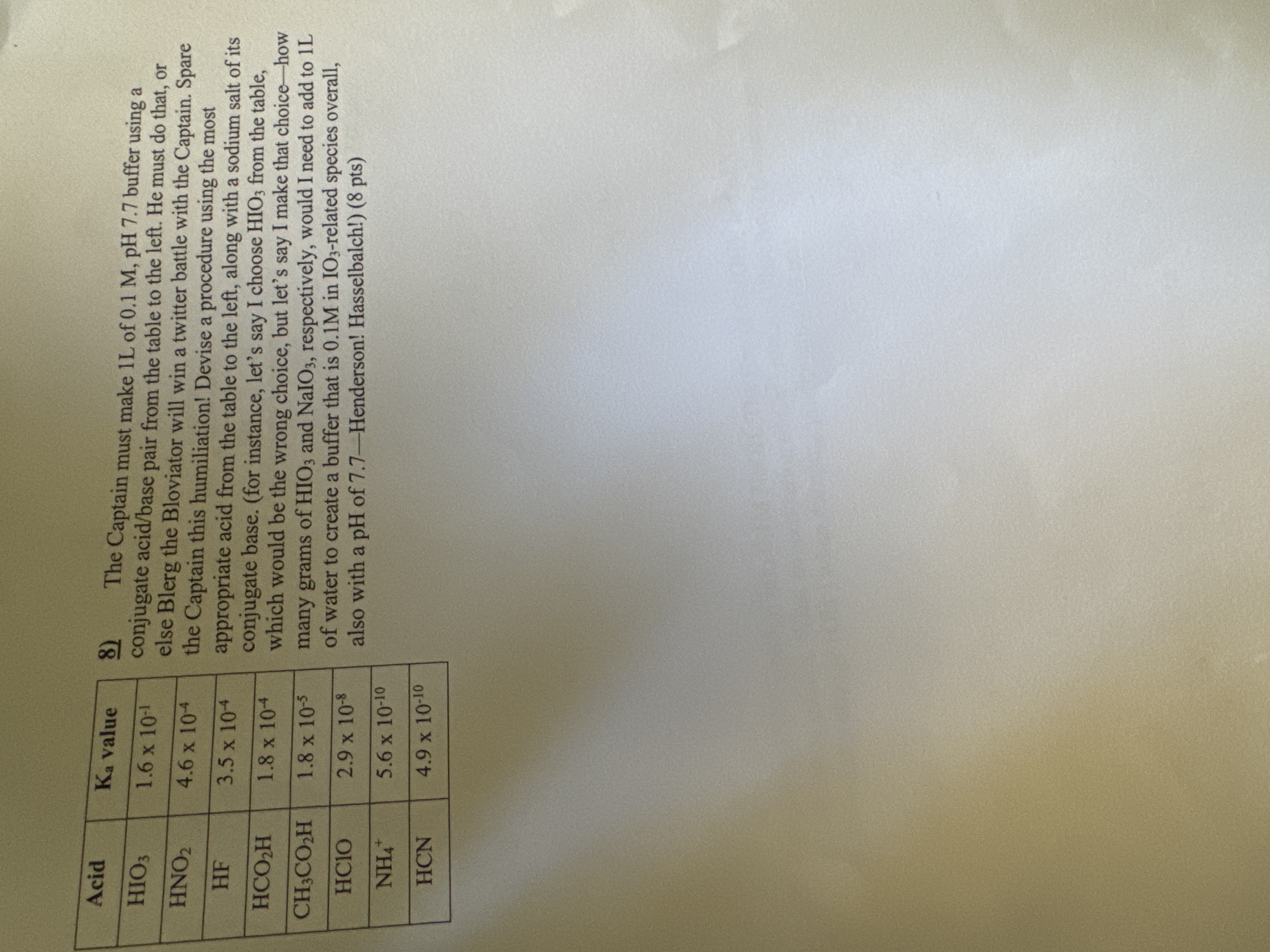
The Captain must make 1 L of 0 . 1 M , p H 7 . 7 buffer using a conjugate acid / base pair
The Captain must make of buffer using a
conjugate acidbase pair from the table to the left. He must do that, or
else Blerg the Bloviator will win a twitter battle with the Captain. Spare
the Captain this humiliation! Devise a procedure using the most
appropriate acid from the table to the left, along with a sodium salt of its
conjugate base. for instance, let's say I choose from the table,
which would be the wrong choice, but let's say I make that choice how
many grams of and respectively, would I need to add to
of water to create a buffer that is in related species overall,
also with a pH of Henderson! Hasselbalch! pts
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


