Answered step by step
Verified Expert Solution
Question
1 Approved Answer
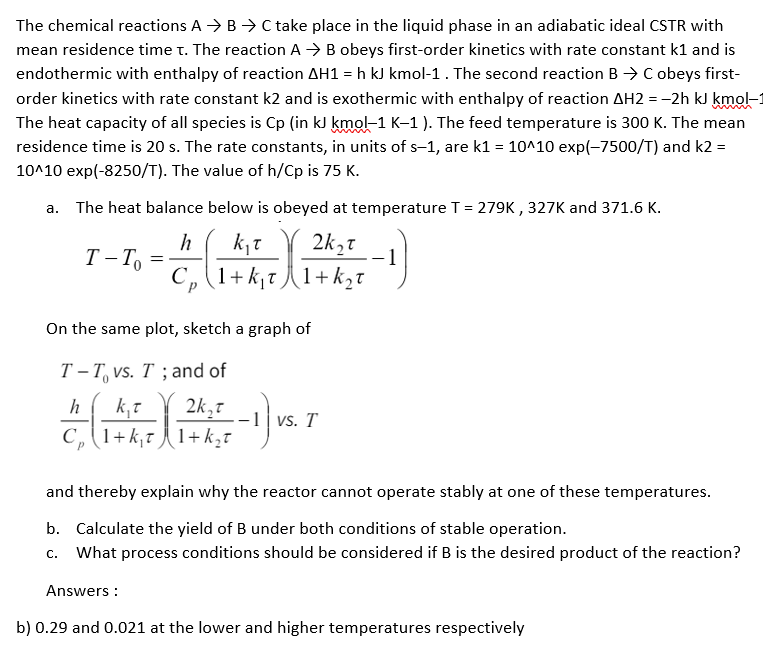
The chemical reactions A B C take place in the liquid phase in an adiabatic ideal CSTR with mean residence time . The reaction A
The chemical reactions take place in the liquid phase in an adiabatic ideal CSTR with
mean residence time The reaction obeys firstorder kinetics with rate constant and is
endothermic with enthalpy of reaction hkJkmol The second reaction obeys first
order kinetics with rate constant and is exothermic with enthalpy of reaction hkJkmol
The heat capacity of all species is in kJ kmol K The feed temperature is The mean
residence time is The rate constants, in units of are exp and
exp The value of is
a The heat balance below is obeyed at temperature and
On the same plot, sketch a graph of
; and
and thereby explain why the reactor cannot operate stably at one of these temperatures.
b Calculate the yield of under both conditions of stable operation.
c What process conditions should be considered if is the desired product of the reaction?
Answers:
b and at the lower and higher temperatures respectively
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


