Question
The curves shown below were obtained when two equal volumes of hydrogen peroxide of same concentration were allowed to decompose separately in one case,
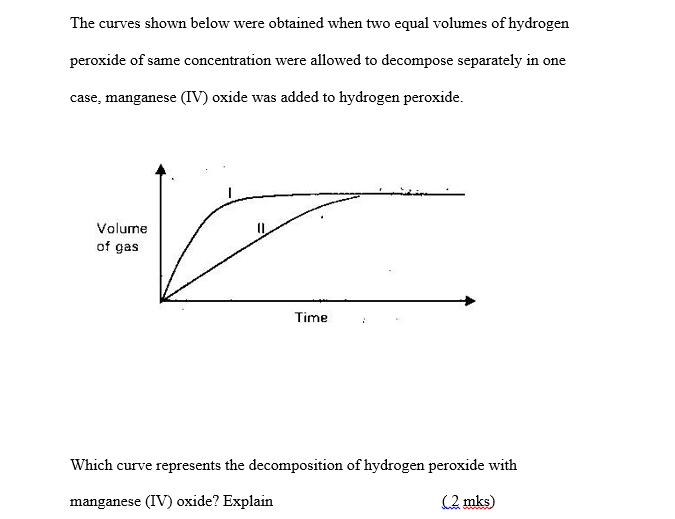
The curves shown below were obtained when two equal volumes of hydrogen peroxide of same concentration were allowed to decompose separately in one case, manganese (IV) oxide was added to hydrogen peroxide. Volume of gas Time Which curve represents the decomposition of hydrogen peroxide with manganese (IV) oxide? Explain (2 mks)
Step by Step Solution
3.42 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
Based on the graph it appears that Curve 2 represents the decomposition of hy...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Cambridge International AS And A Level Chemistry Coursebook
Authors: Lawrie Ryan, Roger Norris
2nd Edition
1316637735, 978-1316637739
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



