Question
The decomposition of urea in 0.1 M of HCl occurs according to the reaction NH,CONH, +2H,02NH; +CO} The first-order rate constant for this reaction
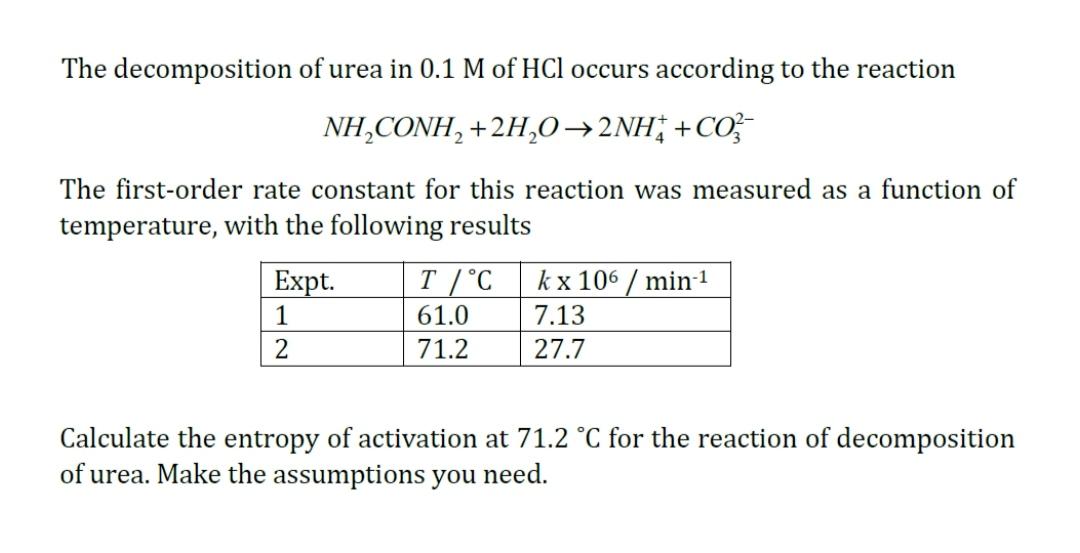
The decomposition of urea in 0.1 M of HCl occurs according to the reaction NH,CONH, +2H,02NH; +CO} The first-order rate constant for this reaction was measured as a function of temperature, with the following results Expt. T /C kx 106 / min-1 1 61.0 7.13 71.2 27.7 Calculate the entropy of activation at 71.2 C for the reaction of decomposition of urea. Make the assumptions you need.
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physical Chemistry
Authors: Thomas Engel, Philip Reid
3rd edition
805338423, 080533842X, 978-0321812001
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



