Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The deuterium isotope (2H or D) has nearly identical reactivity as hydrogen (1H). Consequently, the one-step elimination from (R,R)-2-bromo-3-deuterobutane yields two different disubstituted alkene products.
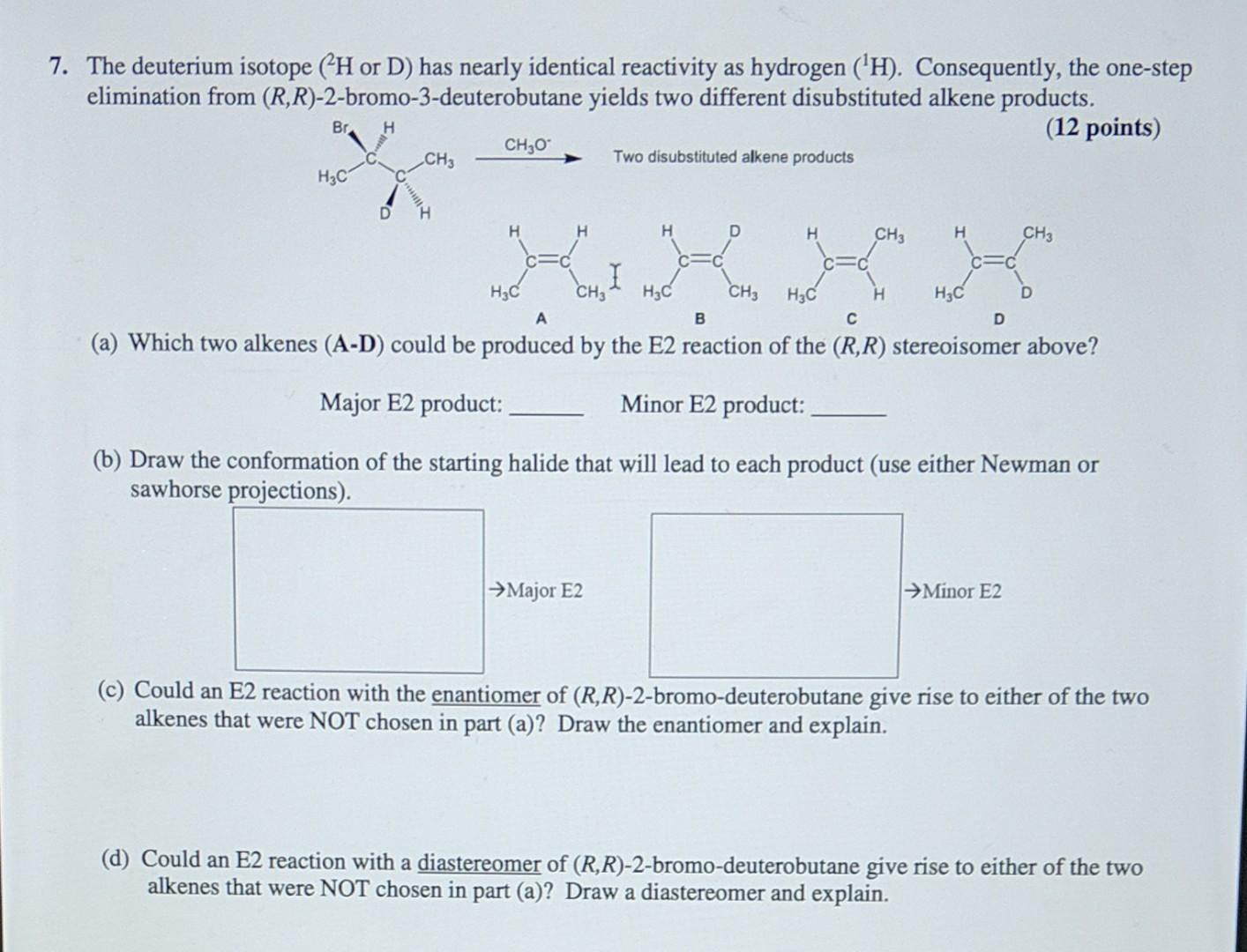
The deuterium isotope (2H or D) has nearly identical reactivity as hydrogen (1H). Consequently, the one-step elimination from (R,R)-2-bromo-3-deuterobutane yields two different disubstituted alkene products. (12 points) Two disubstituted alkene products (a) Which two alkenes (A-D) could be produced by the E2 reaction of the (R,R) stereoisomer above? Major E2 product: Minor E2 product: (b) Draw the conformation of the starting halide that will lead to each product (use either Newman or sawhorse projections). (c) Could an E2 reaction with the enantiomer of (R,R)-2-bromo-deuterobutane give rise to either of the two alkenes that were NOT chosen in part (a)? Draw the enantiomer and explain. (d) Could an E2 reaction with a diastereomer of (R,R)2-bromo-deuterobutane give rise to either of the two alkenes that were NOT chosen in part (a)? Draw a diastereomer and explain
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


